[ad_1]
Characterization of mPDA@CuO2 NRs
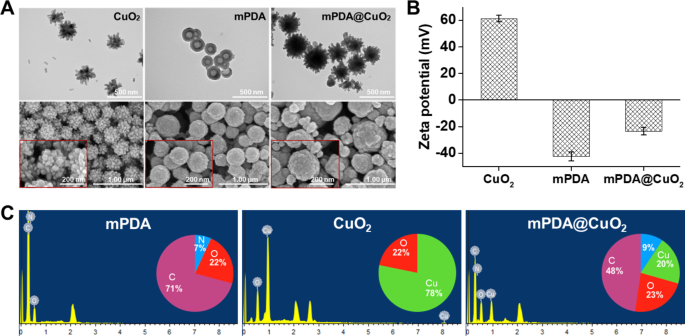
CuO2 clusters had been initially synthesized via the response of copper(II) chloride (CuCl2), H2O2, and alkaline Tris buffer (pH 8.5) at room temperature for 30 min. The ensuing CuO2 clusters served as a template for the preparation of mPDA@CuO2 NRs. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) pictures revealed that the CuO2 clusters exhibited a particular urchin-like form, with a median particle dimension of 260.4 ± 15 nm, indicating their composition of a number of CuO2 clusters (Fig. 1A, left). Then again, mPDA displayed a spherical, hole, and easy morphology, with a median particle dimension of 184.0 ± 10 nm (Fig. 1A, center). Upon the formation of mPDA@CuO2 NRs, the central portion of the unique CuO2 clusters was coated with mPDA, whereas the uncoated outer half retained a meteor hammer form, with a median particle dimension of 283 ± 16 nm (Fig. 1A, proper). Zeta potential evaluation demonstrated that the floor potential of CuO2 clusters was 61.3 ± 2.5 mV, which modified to -23.5 ± 2.8 mV upon coating with mPDA (-42.5 ± 3.6 mV). This modification may be attributed to the deprotonation of phenol teams in mPDA, leading to a negatively charged floor at impartial pH (Fig. 1B). These outcomes affirm the profitable coating of mPDA onto the floor of CuO2 clusters, resulting in the formation of mPDA@CuO2 NRs. Vitality-dispersive X-ray spectroscopy (EDS) additional supported the profitable preparation of mPDA@CuO2 NRs. The basic composition evaluation indicated that mPDA predominantly consisted of carbon (C) (71%), oxygen (O) (22%), and nitrogen (N) (7%), whereas CuO2 clusters was primarily composed of copper (Cu) (78%) and oxygen (O) (22%). After coating mPDA onto CuO2 clusters, the noticed parts had been carbon (C) (48%), nitrogen (N) (9%), copper (Cu) (20%), and oxygen (O) (23%), confirming the profitable preparation of mPDA@CuO2 NRs with roughly 20% of CuO2 clusters remaining uncoated with mPDA (Fig. 1C).
Characterization of synthesized supplies. (A) Transmission electron microscopy (TEM) pictures and scanning electron microscopy (SEM) pictures of CuO2 clusters, mPDA NPs, and mPDA@CuO2 NRs. (B) Zeta potential of CuO2 clusters, mPDA NPs, and mPDA@CuO2 NRs. The values are expressed as means ± SD (n = 3). (C) Elemental evaluation of CuO2 clusters, mPDA NPs, and mPDA@CuO2 NRs
Acid-induced •OH era from mPDA@CuO2 NRs
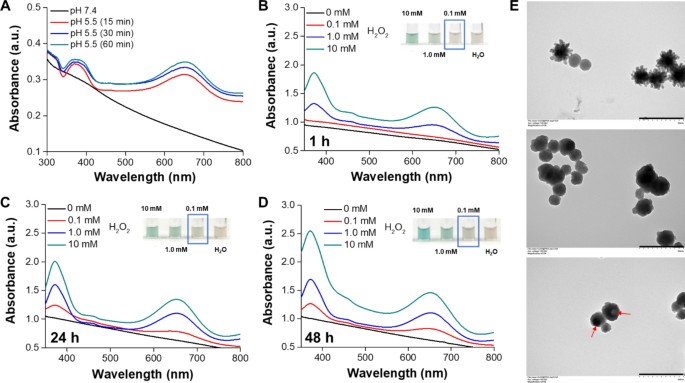
In an acidic setting, the dissociation of CuO2 clusters into Cu2+ and H2O2, together with the next Fenton-type response between these dissociation merchandise, generates •OH for most cancers chemodynamic remedy (CDT). Nonetheless, the speedy decomposition of CuO2 clusters to Cu2+and H2 O2 in an acidic setting limits the period of CDT. From the leads to Fig. S1, a discernible shade change in TMB to a blue-green hue, exhibiting a definite absorbance peak at roughly 650 nm, was noticed when the CuO2 NRs had been incubated in an acidic resolution for 30 min, in comparison with mPDA@CuO2 NRs. This means that the coating of mPDA can impede the speedy decomposition of CuO2 NRs. To handle this, we developed mPDA-coated CuO2 NPs (mPDA@CuO2 NRs) with acid-triggered degradation functionality to stop their speedy decomposition within the acidic tumor microenvironment. To display the acid-triggered H2O2 self-supplying means of mPDA@CuO2 NRs, we suspended the NRs in an acidic resolution (pH 5.5) with out the addition of H2O2 and incubated them for various time intervals. As anticipated, a noticeable shade change from TMB to a blue-green shade with a definite absorbance peak at roughly 650 nm was noticed after 15 min of incubation beneath delicate acidic (pH 5.5) situations, however not beneath impartial (pH 7.4) situations (Fig. 2A). These findings affirm that the mPDA@CuO2 NRs possess acid-responsive traits, permitting them to supply Fenton catalytic Cu2+ and H2O2 for self-supplying CDT within the acidic tumor microenvironment [29]. Subsequently, we carried out an preliminary pretreatment of the mPDA@CuO2 NRs by resuspending them in an acidic resolution (pH 5.5) for 1–48 h to etch away the outer CaO2 clusters layer and mPDA coating layer of the mPDA@CuO2 NRs. In consequence, the spikes on the floor of mPDA@CuO2 NRs disappeared after 1 h incubation, inflicting a morphological transition from an urchin-like form to a sphere-like form (Fig. 2E, center). The ensuing mPDA@CuO2 NRs had been then blended with completely different concentrations (0–10 mM) of H2 O2. The outcomes depicted in Fig. 2B demonstrated that the mPDA@CuO2 NRs had been able to producing enough •OH to oxidize TMB solely within the presence of excessive concentrations of H2 O2 (above 1 mM). No vital peak at 650 nm was noticed when the H2 O2 focus was under 1 mM in the course of the preliminary 1 h. Notably, after 24 h of incubation within the acidic resolution (pH 5.5), the mPDA construction progressively loosened, resulting in the formation of a hole sphere-like form (Fig. 2E, backside). This structural change allowed for extra CuO2 clusters to decompose into Cu2+ and H2O2 within the acidic setting, leading to enough •OH era to oxidize TMB even at decrease H2O2 concentrations (0.1 mM, mimicking the tumor microenvironment) (Fig. 2C&D). The internal CuO2 clusters layer underwent Fenton response, producing •OH. The absorption peaks at 370/650 nm elevated with the H2O2 focus and the period of mPDA immersion within the resolution, indicating that •OH era was straight proportional to the H2O2 focus. This remark is probably going because of the gradual loosening of the mPDA construction in a impartial setting and its speedy loosening in an acidic setting. These outcomes display that the mPDA coating successfully prevents the speedy decomposition of CuO2 clusters into Cu2+ and H2O2 in a impartial setting, thus decreasing potential unwanted side effects in regular tissues.
(A) Colorimetric detection of •OH generated by mPDA@CuO2 NRs at completely different pH values based mostly on the TMB assay. UV-vis spectra and images (inset) of TMB aqueous resolution incubated with completely different concentrations of H2O2 within the presence of mPDA@CuO2 NRs (100 µg/mL) pretreated in an acidic resolution for (B) 1 h, (C) 24 h, and (D) 48 h. (E) TEM pictures of mPDA@CuO2 NRs (prime), mPDA@CuO2 NRs handled in an acidic resolution for 1 h (center), and mPDA@CuO2 NRs handled in an acidic resolution for twenty-four h (backside). Scale bar = 500 nm
In vitro ROS era and CDT efficacy
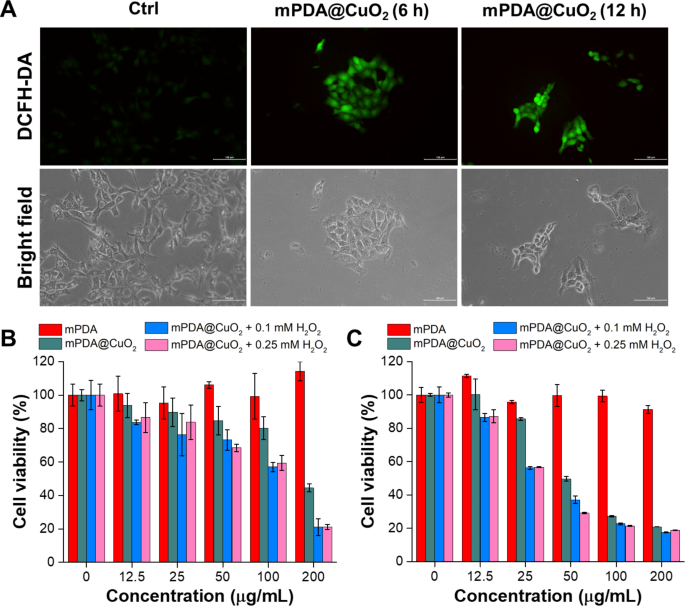
pH-sensitive mPDA@CuO2 NRs can bear decomposition inside most cancers cells following endocytosis, resulting in a Fenton-like response between the launched Cu2+ and H2O2 within the acidic setting of endosomes, ensuing within the era of •OH. To guage the manufacturing of •OH by mPDA@CuO2 NRs on the mobile degree, we utilized 2’,7’-dichlorofluorescin diacetate (DCFH-DA) as a fluorescent indicator of reactive oxygen species (ROS). Elevated fluorescence depth signifies a better degree of •OH era. The outcomes depicted in Fig. 3A display that 4T1 most cancers cells incubated with mPDA@CuO2 NRs for six h exhibited considerably increased inexperienced fluorescence (Fig. 3A, center) in comparison with the untreated management group (Fig. 3A, left). Furthermore, the ROS-associated inexperienced fluorescence sign was nonetheless observable even after incubating the cells with mPDA@CuO2 NRs for over 12 h (Fig. 3A, proper). This remark means that mPDA@CuO2 NRs effectively generate •OH inside most cancers cells, permitting for a sustained two-stage Fenton-like response and longer-lasting chemodynamic remedy (CDT).
(A) Fluorescence and bright-field pictures of DCFH-DA-stained 4T1 most cancers cells after publicity to mPDA@CuO2 NRs for six and 12 h. The size bar represents 100 μm. (B) In vitro CDT efficiency of mPDA@CuO2 NRs after 24 h of incubation with MDA-MB-468 cells within the presence of various concentrations of H2O2. The values are expressed as means ± SD (n = 8). (C) In vitro CDT efficiency of mPDA@CuO2 NRs after 24 h of incubation with 4T1 cells within the presence of various concentrations of H2O2. The values are expressed as means ± SD (n = 8)
Subsequently, we quantitatively assessed the in vitro CDT effectivity of mPDA@CuO2 NRs in opposition to most cancers cells (MDA-MB-468 cells and 4T1 cells) utilizing the XTT assay. As depicted in Fig. 3B, MDA-MB-468 cells handled with 25 µg/mL of mPDA@CuO2 NRs for twenty-four h exhibited a cell viability of roughly 85.6%. Nonetheless, when an extra H2O2 focus of 0.1 mM was launched into the tradition medium, the cell viability considerably decreased to 56.2%. This lower in cell viability may be attributed to the truth that the low focus of mPDA@CuO2 NRs (25 µg/mL) was inadequate to generate an enough quantity of H2O2, leading to an insufficient manufacturing of •OH. Curiously, when the focus of mPDA@CuO2 NRs was elevated to 50 µg/mL, extra MDA-MB-468 cells had been killed. Intriguingly, mPDA@CuO2 NRs exhibited decrease toxicity in direction of 4T1 cells, which may be attributed to their speedy proliferation fee (Fig. 3C). Nonetheless, even at a focus of 200 µg/mL, the cell viability of 4T1 cells decreased to 44.5%. Moreover, when an extra H2O2 focus of 0.1 mM was launched, the cell viability additional decreased to twenty.9%. These outcomes point out that MDA-MB-468 cells had been extra delicate to mPDA@CuO2 NRs, with an IC50 worth of 51.9 µg/mL, whereas 4T1 cells had an IC50 worth of 190.7 µg/mL. Furthermore, when H2O2 was launched at a focus much like the tumor microenvironment (0.1 mM), the IC50 values considerably decreased to 25.6 µg/mL for MDA-MB-468 cells and 106.9 µg/mL for 4T1 cells. These findings additional affirm that mPDA@CuO2 NRs, which function enhanced chemodynamic nanoagents with self-supplied H2 O2, exhibit potent anticancer exercise. Furthermore, their cytotoxicity effectivity in opposition to TNBC cells is concentration-dependent.
Investigation of PD-L1 and CD24 expression in TNBC cells
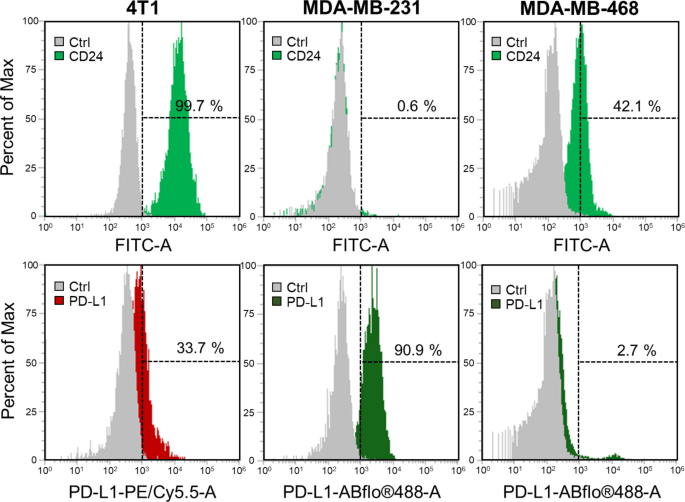
On this research, our goal is to mix chemodynamic remedy (CDT) with checkpoint blockade immunotherapy (CBIT) for the therapy of triple-negative breast most cancers (TNBC). To evaluate the expression ranges of immune checkpoint proteins in TNBC cells, particularly PD-L1 and CD24, we performed circulate cytometry evaluation on two varieties of human TNBC cells (MDA-MB-231, and MDA-MB-468) in addition to one mouse TNBC cell line (4T1 cells). The information offered in Fig. 4 display that, among the many examined cell strains, solely 4T1 cells exhibit concurrent expression of each PD-L1 and CD24, with the potential for CD24 expression reaching as much as 99.7%. Conversely, MDA-MB-231 and MDA-MB-468 cells completely specific both PD-L1 or CD24. Extra particularly, MDA-MB-231 cells show a excessive expression degree of PD-L1 (90.9%) with out notable expression of CD24 (0.6%). Though MDA-MB-468 cells do manifest expression of each CD24 (42.1%) and PD-L1 (2.7%), the extent of PD-L1 expression is extraordinarily low, verging on negligible. From the outcomes, it’s evident that not all cells will specific each PD-L1 and CD24 concurrently. Primarily based on these findings, we endeavored to immobilize AbPD−L1 and AbCD24 concurrently on the floor of mPDA@CuO2 NRs. This led to the creation of dAbPD−L1/CD24 -mPDA@CuO2 NRs, permitting for a broader and extra complete utility of CBIT for TNBC. This technique facilitates the combination of CDT and twin CBIT in treating TNBC.
Quantification of AbPD−L1 and AbCD24 immobilization
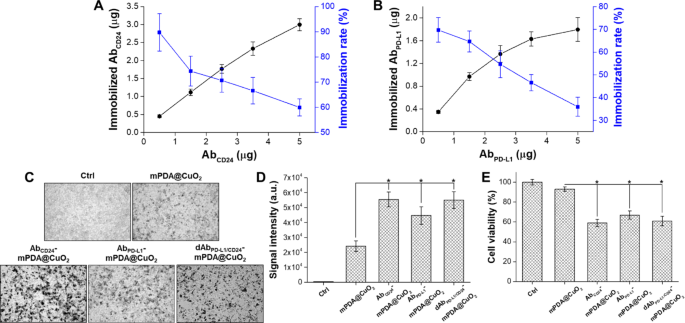
The portions of AbPD−L1 and AbCD24 sure to mPDA@CuO2 NRs had been decided by analyzing the unbound antibodies within the supernatant utilizing an ELISA technique. The quantity of AbCD24 immobilized on 100 µg of mPDA@CuO2 NRs elevated with increased concentrations of added AbCD24. At 5 µg of added AbCD24, roughly 3.0 ± 0.2 µg of AbCD24 was sure to 100 µg of mPDA@CuO2 NRs, leading to a conjugation fee of 59.9 ± 3.4% (Fig. 5A). Then again, the amount of immobilized AbPD−L1 reached saturation (1.6 ± 0.1 µg AbPD−L1/100 µg mPDA@CuO2 NRs) at 3.5 µg of added AbPD−L1, with a conjugation fee of 46.6 ± 3.6%. Though the quantity of immobilized AbPD−L1 may very well be elevated to 1.8 ± 0.2 µg with 5 µg of added AbPD−L1, the conjugation fee considerably decreased to 35.9 ± 4.2% (Fig. 5B). The lowered conjugation fee could be because of the majority of the floor space being occupied by AbCD24. This might probably be attributed to the upper affinity of AbCD24 with mPDA, leading to a decrease conjugation fee for AbPD−L1 in comparison with AbCD24. Taken collectively, these outcomes display that roughly 3.0 ± 0.2 µg of AbCD24 and 1.6 ± 0.1 µg of AbPD−L1 may be immobilized on 100 µg of mPDA@CuO2 NRs.
(A) Evaluation of the optimum immobilization fee of AbCD24 on mPDA@CuO2 NRs utilizing ELISA. (B) Evaluation of the optimum immobilization fee of AbPD−L1 on AbCD24-mPDA@CuO2 NRs utilizing ELISA. (C) Vivid-field pictures of 4T1 cells handled with PBS (Ctrl), mPDA@CuO2 NRs, AbCD24-mPDA@CuO2 NRs, AbPD−L1-mPDA@CuO2 NRs, and dAbPD−L1/CD24-mPDA@CuO2 NRs. The black dots point out mPDA@CuO2 NRs. (D) The information are offered because the imply depth of black dots, which was calculated from the pictures in (C). The values are expressed as means ± SD (n = 3). Asterisks point out a major distinction between the mPDA@CuO2 NRs and AbCD24-mPDA@CuO2 NRs, AbPD−L1-mPDA@CuO2 NRs, dAbPD−L1/CD24-mPDA@CuO2 NRs teams (Scholar’s t-test, *p ≤ 0.05). (E) The impact of residual mPDA@CuO2 NRs, AbCD24-mPDA@CuO2 NRs, AbPD−L1-mPDA@CuO2 NRs, and dAbPD−L1/CD24-mPDA@CuO2 NRs on 4T1 cell viability measured by XTT assay after an extra 24 h of incubation. The values are expressed as means ± SD (n = 3). Asterisks point out a major distinction between the mPDA@CuO2 NRs and AbCD24-mPDA@CuO2 NRs, AbPD−L1-mPDA@CuO2 NRs, dAbPD−L1/CD24-mPDA@CuO2 NRs teams (Scholar’s t-test, *p ≤ 0.05)
In vitro cell focusing on efficacy and cytotoxicity
We examined the binding effectivity of mPDA NPs, AbCD24-mPDA@CuO2 NRs, and AbPD−L1-mPDA@CuO2 NRs to 4T1 cells by incubating the supplies with the cells for two h, adopted by washing with contemporary DMEM medium to take away unbound supplies. As proven in Fig. 5C&D, AbCD24 -mPDA@CuO2 NRs (sign depth: 5.6 × 104), AbPD−L1 -mPDA@CuO2 NRs (sign depth: 4.5 × 104), and dAbPD−L1/CD24 -mPDA@CuO2 NRs (sign depth: 5.5 × 104) all demonstrated considerably increased attachment to the cell membrane in comparison with mPDA@CuO2 NRs (sign depth: 2.4 × 104), with roughly 1.9 ~ 2.4-fold increased sign depth. Nonetheless, the quantity of dAbPD−L1/CD24 -mPDA@CuO2 NRs hooked up to the 4T1 cells was not vital increased than that of AbPD−L1 -mPDA@CuO2 NRs or AbCD24 -mPDA@CuO2 NRs. It’s because their complete antibody content material is analogous. But, since dAbPD−L1/CD24 -mPDA@CuO2 NRs comprise each AbPD−L1 and AbCD24, its benefit lies in its means to focus on cells extra successfully, so long as the cells specific both PD-L1 or CD24. To research the influence of focusing on on CDT, the 4T1 cells had been additional incubated in contemporary DMEM containing 0.1 mM H2O2 at 37 °C for twenty-four h after eradicating unbound supplies (Fig. 5E). The cell viability was 93 ± 2.3% for the mPDA@CuO2 NRs group, however considerably decreased to 59 ± 3.7% for the AbCD24 -mPDA@CuO2 NRs group, 63 ± 4.1% for the AbPD−L1 -mPDA@CuO2 NRs group, and 61 ± 4.7% for the dAbPD−L1/CD24 -mPDA@CuO2 NRs group in comparison with the management group (handled with 0.1 mM H2 O2 ). These outcomes point out that the modification of mPDA@CuO2 NRs with AbCD24 and AbPD−L1 successfully targets 4T1 cells, resulting in enhanced CDT efficacy and blocking of CD24-Siglec-10 and PD-L1-PD1 signaling pathways.
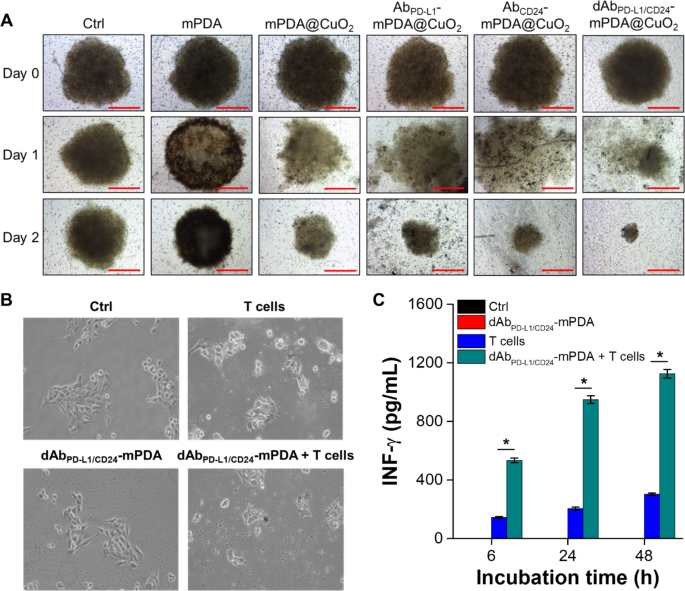
We additional investigated the cell focusing on and anticancer effectivity of mPDA NPs, mPDA@CuO2 NRs, AbCD24-mPDA@CuO2 NRs, AbPD−L1-mPDA@CuO2 NRs, and dAbPD−L1/CD24-mPDA@CuO2 NRs utilizing a cultured three-dimensional tumor spheroid by using 4T1 cells as a cell mannequin. The 4T1 tumor spheroid was fashioned in an ultra-low attachment round-bottomed plate after which transferred to an agarose-coated flat-bottomed 96-well plate. The spheroids had been pretreated with completely different supplies for six h, after which transferred to a brand new properly and cultured with contemporary DMEM for an extra one or two days. As proven in Fig. 6A, after someday of incubation, the 4T1 tumor spheroid within the mPDA@CuO2 NRs, AbCD24-mPDA@CuO2 NRs, AbPD−L1-mPDA@CuO2 NRs, and dAbPD−L1/CD24-mPDA@CuO2 NRs teams started to disintegrate and appeared looser in comparison with the group handled with mPDA NPs. Moreover, the amount of the 4T1 tumor spheroid handled with AbCD24-mPDA@CuO2 NRs or AbPD−L1-mPDA@CuO2 NRs was smaller than the management group and the mPDA@CuO2 NRs-treated group after two days of incubation. This means that AbCD24-mPDA@CuO2 NRs or AbPD−L1-mPDA@CuO2 NRs effectively hooked up to the 4T1 tumor spheroid, resulting in H2O2 self-supplying CDT. Notably, the amount of the 4T1 tumor spheroid handled with dAbPD−L1/CD24 -mPDA@CuO2 NRs was the smallest (241.3 ± 23.8 μm) among the many therapy teams (1002.7 ± 68.1 μm for mPDA NPs and 434.7 ± 105.8 μm for mPDA@CuO2 NRs) after two days of incubation (Fig. S2). That is seemingly because of the enhanced attachment of dAbPD−L1/CD24-mPDA@CuO2 NRs to the 4T1 tumor spheroid, as dAbPD−L1/CD24-mPDA@CuO2 NRs can bind to CD24 and PD-L1 on the cell membrane concurrently, enabling extremely environment friendly H2O2 self-supplying CDT on the floor of the 4T1 tumor spheroid.
4T1 cells had been cultured in an ultra-low attachment plate to kind tumor spheroids, adopted by therapy with completely different supplies within the tradition medium over time. (A) The quantity of 4T1 tumor spheroids after therapy with PBS (Ctrl), mPDA NPs, mPDA@CuO2 NRs, AbPD−L1-mPDA@CuO2 NRs, AbCD24-mPDA@CuO2 NRs, and dAbPD−L1/CD24-mPDA@CuO2 NRs. Scale bar = 500 μm. (B) In vitro CBIT effectivity examined by blocking PD-L1–PD1 signaling between most cancers cells and T cells utilizing dAbPD−L1/CD24-mPDA NPs. (C) Era of IFN-γ from T cells after co-culturing with 4T1 cells handled with dAbPD−L1/CD24-mPDA NPs. The values are expressed as means ± SD (n = 3). Asterisks point out a major distinction between the T cells handled and dAbPD−L1/CD24-mPDA NPs + T cells handled teams (Scholar’s t-test, *p ≤ 0.05). Word: The absence of black and pink bars is because of their values being extraordinarily low, nearly equal to 0
Examine of in vitro CBIT effectivity
The expression degree of PD-1/PD-L1 in tumor tissue is thought to be related to scientific outcomes, tumor metastasis, and total survival in numerous cancers, together with melanoma, breast most cancers, and pancreatic most cancers. On this research, we aimed to develop dAbPD−L1/CD24-mPDA@CuO2 NRs to reinforce the immune cells’ means to detect and get rid of most cancers cells. To confirm the efficacy of dAbPD−L1/CD24 -mPDA@CuO2 NRs in checkpoint blockade immunotherapy (CBIT), we executed profitable blockage of PD-L1 and CD24 on 4T1 cells. This was performed by pretreating the cells with dAbPD−L1/CD24 -mPDA@CuO2 NRs for six h and subsequently staining for residual, unblocked PD-L1 and CD24. The outcomes revealed that roughly 20.2 ± 4.8% of CD24 and 38.5 ± 6.2% of PD-L1 on the 4T1 cell floor had been successfully blocked by dAbPD−L1/CD24 -mPDA@CuO2 NRs (Fig. S3). Following this, the cells had been co-cultured with mouse T cells for an extra 48 h. The outcomes proven in Fig. 6B revealed that the morphology of 4T1 cells exhibited slight variations after co-culturing with T cells in comparison with the management group and the group handled with dAbPD−L1/CD24-mPDA NPs alone. This may be attributed to the PD-L1-PD1 signaling between 4T1 cells and T cells, which transmits a “don’t eat me” sign to T cells [30]. Nonetheless, when 4T1 cells had been pretreated with dAbPD−L1/CD24-mPDA NPs to dam PD-L1-PD1 signaling and reactivate T cells, the T cells efficiently attacked and destroyed the 4T1 cells. Moreover, we investigated the concentrations of IFN-γ secreted by T cells, as activated CD8 + cytotoxic T lymphocytes (CTLs) exert their antitumor results by releasing IFN-γ, tumor necrosis factor-alpha (TNF-α), and different cytotoxins [31]. As depicted in Fig. 6C, solely a minimal quantity of IFN-γ (302.3 ± 8.1 pg/mL at 48 h of co-culture) was secreted when T cells had been straight co-cultured with 4T1 cells. Nonetheless, when T cells had been co-cultured for 48 h with 4T1 cells that had been pretreated with dAbPD−L1/CD24-mPDA NPs, the focus of secreted IFN-γ considerably elevated to 1125.3 ± 28.9 pg/mL. These outcomes affirm that dAbPD−L1/CD24-mPDA NPs successfully block PD-L1-PD1 signaling between 4T1 cells and T cells, reactivate T cells, and promote the secretion of enough IFN-γ to induce most cancers cell demise [32].
dAbPD−L1/CD24-mPDA@CuO2 NRs inhibit Tumor development within the tumor-bearing mice
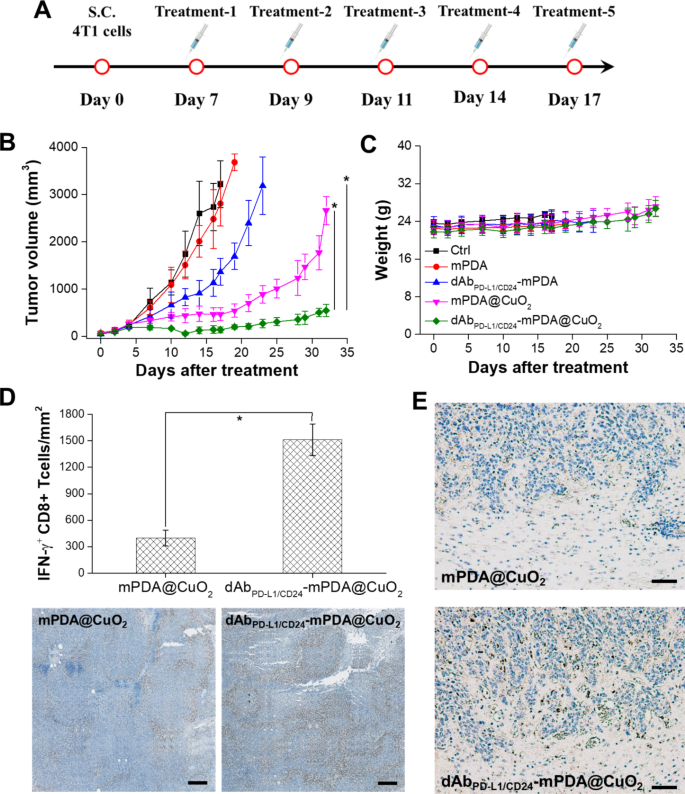
The therapeutic efficacy of dAbPD−L1/CD24-mPDA@CuO2 NRs in TNBC was evaluated by monitoring tumor dimension. When the tumor quantity reached roughly 100 mm3, mice had been handled intratumorally with PBS (management), clean mPDA NPs, dAbPD−L1/CD24 -mPDA NPs, mPDA@CuO2 NRs, and dAbPD−L1/CD24 -mPDA@CuO2 NRs (roughly 4 mg/kg) on day 7, day 9, day 11, day 14, and day 17 after the implantation of 4T1 tumor cells to evaluate the antitumor efficacy (Fig. 7A).
Anti-tumor impact of AbPD−L1/CD24-mPDA@CuO2 NRs in vivo. (A) Therapy protocols assessing H2O2 self-supplying CDT + CBIT by intratumoral injection of dAbPD−L1/CD24-mPDA@CuO2 NRs. (B) Time-dependent tumor development curves in 4T1 tumor-bearing mice after numerous therapies with intratumoral injections. The values are expressed as means ± SD (n = 8). Asterisks point out a major distinction between the AbPD−L1/CD24-mPDA@CuO2 NRs handled group and the AbPD−L1/CD24-mPDA NPs and mPDA@CuO2 NRs handled teams (Scholar’s t-test, *p ≤ 0.05). (C) Physique weight of 4T1 tumor-bearing mice in numerous teams after therapies. The values are expressed as means ± SD (n = 8). (D) Protein expression degree of IFN-γ in tumor tissues after therapy to dam the PD-L1–PD1 signaling between most cancers cells and T cells utilizing AbPD−L1/CD24-mPDA@CuO2 NRs (the underside inset exhibits a corresponding digital picture of immunohistochemistry). The values are expressed as means ± SD (n = 3). Asterisks point out a major distinction (Scholar’s t-test, *p ≤ 0.05). Scale bar = 200 μm. (E) Immunohistochemical evaluation of immune responses, specializing in CD68 (infiltrating macrophages) in tumor tissues from mice handled with mPDA@CuO2 NRs and dAbPD−L1/CD24 -mPDA@CuO2 NRs. Scale bar = 500 μm
As depicted in Fig. 7B, therapy with mPDA NPs didn’t exhibit vital anti-tumor effectivity because of the lack of H2O2 self-supplying CDT and T-cell activation. Nonetheless, at day 17, mice handled with dAbPD−L1/CD24-mPDA NPs confirmed a discount in tumor quantity (1362.9 ± 284.8 mm3) in comparison with the management group (3218.5 ± 498.6 mm3) and mPDA NPs handled group (2809.5 ± 477.5 mm3). This discount may be attributed to the binding of dAbPD−L1/CD24-mPDA NPs to CD24 and PD-L1 molecules on tumor cells, which might goal cytotoxic T lymphocytes in opposition to tumor cells and induce cytokine manufacturing (e.g., IFN-γ). Nonetheless, after 17 days of CBIT therapy, the tumor started to develop quickly, probably because of the incomplete blockade of PD-L1 on the tumor cell membrane by dAbPD−L1/CD24-mPDA NPs. Due to this fact, combining CBIT with different therapy methods (e.g., chemotherapy, photothermal remedy, and CDT) is critical to attain full ablation of TNBC. Moreover, Fig. 7B demonstrates that tumor development may very well be successfully inhibited (463.1 ± 134.9 mm3 at day 17) when mice acquired mPDA@CuO2 NRs for H2O2 self-supplying CDT. Nonetheless, tumor recurrence was noticed after 21 days of H2O2 self-supplying CDT therapy, seemingly because of the revival of non-affected tumor cells attributable to inadequate era of •OH.
On this research, we built-in CBIT with H2O2 self-supplying CDT to attain higher tumor inhibition. Our findings revealed that dAbPD−L1/CD24-mPDA@CuO2 NRs inhibited tumor development in most mice, and no vital tumor recurrence was noticed till day 32 (545.2 ± 129.6 mm3). This means that the binding of dAbPD−L1/CD24-mPDA@CuO2 NRs to CD24/PD-L1 on the floor of 4T1 tumor cells resulted within the era of •OH, T-cell activation, and secretion of IFN-γ, exhibiting a synergistic impact of CBIT and H2O2 self-supplying CDT. Lastly, the potential in vivo toxicity of dAbPD−L1/CD24-mPDA@CuO2 NRs therapy was evaluated, and no vital variations in physique weight among the many therapy teams had been noticed (Fig. 7C), indicating negligible off-target unwanted side effects for all therapies.
To additional affirm the flexibility of the dAbPD−L1/CD24-mPDA@CuO2 NRs to reactivate the T-cells by way of blocking PD-L1–PD1 signaling, we analyzed the secretion degree of IFN-γ within the tumor setting after therapy with immunohistochemistry. As proven in Fig. 7D, increased IFN-γ secretion was noticed after the dAbPD−L1/CD24-mPDA@CuO2 NRs therapy. Semiquantification confirmed that the tumors handled with mPDA@CuO2 NRs discovered low-density of IFN-γ+ CD8 T cells (398.5 ± 89.3 IFN-γ+ CD8 T cells/mm2) within the tumor space. In distinction, the tumors handled with the dAbPD−L1/CD24-mPDA@CuO2 NRs confirmed considerably elevated density of IFN-γ+ CD8 T cells (1512.6 ± 178.4 IFN-γ+ CD8 T cells/mm2) within the tumor space. These outcomes may very well be attributed to the excessive tumor accumulation of the dAbPD−L1/CD24-mPDA@CuO2 NRs by way of binding to CD24 and PD-L1 on the 4T1 tumor cells, which might block PD-L1–PD1 signaling to reactivate T-cells and set off IFN-γ secretion. Now we have additionally verified that dAbPD−L1/CD24 -mPDA@CuO2 NRs can reactivate macrophages by blocking CD24–Siglec-10 signaling. This was demonstrated by staining for CD68 in tumor tissues and analyzing them utilizing immunohistochemistry post-treatment. The outcomes, illustrated in Fig. 7E, reveal a considerable presence of CD68 + infiltrating macrophages in tumor tissues handled with dAbPD-L1/CD24-mPDA@CuO2 NRs in comparison with these handled with mPDA@CuO2 NRs. Taking collectively, dAbPD−L1/CD24 -mPDA@CuO2 NRs have the aptitude to dam each PD-L1–PD1 and CD24–Siglec-10 signaling pathways concurrently, reactivating IFN-γ + CD8 T cells and CD68 + infiltrating macrophages in TNBC CBIT.
[ad_2]