[ad_1]
Preparation and characterization of Algs and PB Nanozymes
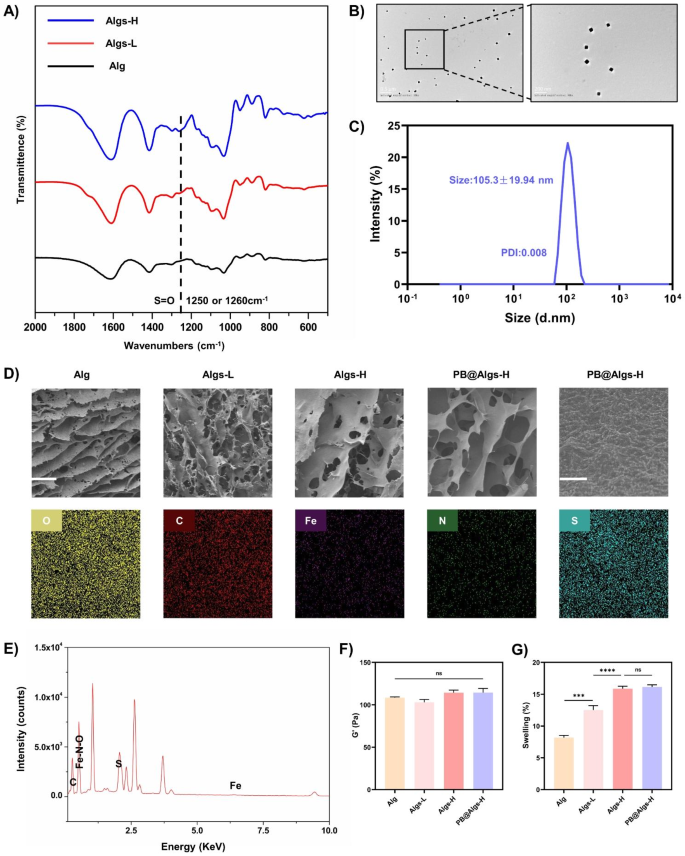
The Fig. 1 confirmed an easy schematic of the preparation strategy of Algs and PB nanozymes. Briefly, Algs was synthesized in line with the beforehand protocol within the literature [20]. Algs with totally different sulfation levels (Algs-L and Algs-H) have been obtained by various the focus of chlorosulfonic acid (HClSO3) as a distinction to the unsulfated Alg. FTIR spectra of Algs-L and Algs-H at 1250 cm− 1 or 1260 cm− 1 confirmed the S = O stretching attribute peak (Fig. 2A). Moreover, we quantitatively analyzed the sulfur content material in Algs-L and Algs-H by elemental evaluation (Determine S1D), reflecting the distinction in sulfation diploma between these two merchandise.
We fabricated the secure PBNPs by a easy hydrothermal methodology within the literature [42]. The UV−vis spectroscopy of PBNPs revealed a robust absorbance peak at ~ 700 nm attributable to the intermetallic cost switch from Fe(II) to Fe(III) (Determine S1C). Transmission electron microscopy (TEM) photos displayed the well-formed homogeneous sq. PBNPs, which possessed excessive monodispersity (Fig. 2B) with common diameter of 54.60 ± 11.96 nm (Determine S2A). And the typical hydrodynamic particle diameter of PBNPs was about 105.3 nm with a slim measurement distribution (polydispersity index: 0.008) (Fig. 2C), and the zeta potential was about − 14.7 mV (Determine S1B), indicating that PBNPs have been nicely dispersed in aqueous media. Good dispersibility of PBNPs is an overriding prerequisite for his or her antioxidant exercise.
Characterization of hydrogels. (A) FTIR spectra of Alg, Algs-L, and Algs-H. Line signifies peaks attribute to S = O stretching at 1250 cm− 1 or 1260 cm− 1. (B) TEM photos of PBNPs. (C) The hydrodynamic particle distribution of PBNPs in aqueous media. (D) SEM photos of hydrogels and ingredient mappings of O, S, Fe, C, and N parts of PB@Algs-H hydrogels. Scale bar: 100 μm and 1 μm. (E) The energy-dispersive X-ray spectrum (EDS) of PB@Algs-H hydrogels. (F) Storage modulus (G’) of hydrogels. (G) % mass swelling of hydrogels after 24 h. (n = 3; imply ± s.d.; ns, not statistically vital; ***p < 0.001, ****p < 0.0001)
Preparation, characterization, and Biocompatibility of Hydrogels
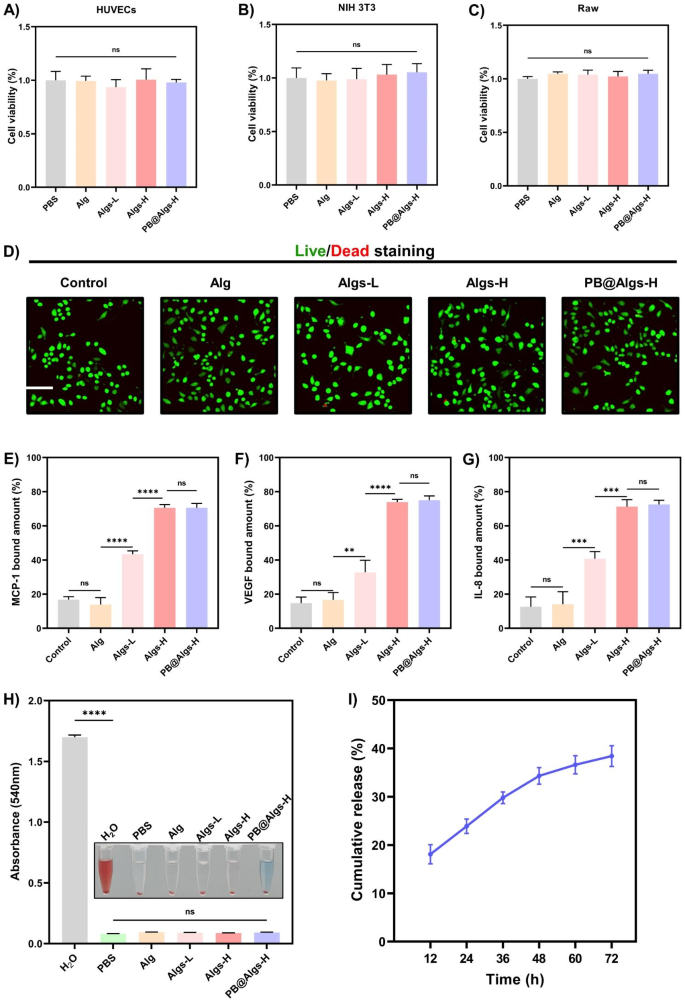
Quite a few research have unveiled that one of many causes for the widespread scientific purposes of hydrogels is that their smooth, porous nature is in step with that of ECM [48,49,50,51], permitting for hydrogel-skin mutual adaptation. Below such circumstances, cells in burn wounds readily migrate and proliferate in response to organic cues with out exterior interference [50,51,52]. To realize a secure hydrogel framework whereas preserving the softness of hydrogels, the focus of Alg or Algs was maintained at 2%. In the meantime, the stiffness of hydrogels was uniformed by adjusting the crosslinking agent focus (Fig. 2F and Determine S2A-E) for excluding their affect on experiment outcomes. And different rheological properties of hydrogels have been systematically examined (Determine S2G-I). Ca2+ is non-toxic to cells in comparison with different crosslinking ions, reminiscent of Pb2+, Cd2+, Ba2+, and so on., and is indispensable for physiological actions of the physique. Accordingly, Ca2+ was chosen because the crosslinking agent, and the Ca2+ concentrations have been 50 mM (Alg), 60 mM (Algs-L), 90 mM (Algs-H), and 85 mM (PB@Algs-H), respectively. Amongst them, the focus of PBNPs within the PB@Algs-H group was 200 µg/ml, and for the reason that ferric ions acted as a partial crosslinker function, the Ca2+ focus within the PB@Algs-H group was barely decrease than that within the Algs-H group. The introduction of sulfate group imparted extra unfavorable cost to Alg and raised the hydrophilicity of Algs hydrogels. As a consequence, the mass swelling ratio of Algs hydrogels was elevated with the rise of sulfate group. And Algs-H hydrogels introduced the best swelling capability of about 16%, whereas the PB@Algs-H and Algs-H teams confirmed no vital distinction in swelling habits (Fig. 2G). The swelling means of hydrogels endows them with absorbing exudate from the wound mattress and fostering the evacuation of necrotic tissue. Scanning electron microscopy (SEM) photos revealed that hydrogels in all teams displayed a porous community construction resembling ECM, and ingredient mapping and EDS confirmed that the doped PBNPs have been uniformly distributed all through the Algs-H polymer community (Fig. 2D and E). The regular launch of PBNPs from the hydrogel system is a vital precondition for his or her antioxidant results. We examined the kinetic properties of in vitro launch of PB@Algs-H hydrogels (Fig. 3I). At 37 °C, PB@Algs-H hydrogels manifested a sustained and managed launch habits, reaching a cumulative launch of roughly 36% after 72 h of incubation. Due to this fact, PBNPs will obtain gradual launch from PB@Algs-H hydrogels and constantly exert antioxidant results after being deployed on burn wounds.
For sensible purposes of hydrogel dressings, distinctive biocompatibility is a elementary requirement [49,50,51]. To evaluate the biocompatibility of hydrogels, we substantiated the in vitro security of hydrogels by the Cell Counting Equipment (CCK-8), dwell/lifeless staining, and a hemolysis assay. Firstly, mouse-derived macrophages (Raw264.7) have been co-cultured with totally different hydrogels, and dwell/lifeless staining assay was executed after 24 h of co-incubation (Fig. 3B). The overwhelming majority of macrophages have been alive (inexperienced fluorescence) and solely few lifeless cells have been noticed (pink fluorescence) within the management, Alg, Algs-L, Algs-H, and PB@Algs-H teams, indicating that the hydrogels might assist cell adhesion and development. The subsequent CCK-8 experiment indicated that cell viability remained consistency with the management group when HUVECs, NIH 3T3, and Raw264.7 cells have been co-cultured with hydrogels for twenty-four h, suggesting that the hydrogels have been freed from cytotoxic results (Fig. 3A-C). Lastly, given the inevitable contact of hydrogels with blood, a hemolysis check was taken to additional consider the hemocompatibility of hydrogels. After incubation with mouse blood, the hydrogel teams didn’t trigger any vital hemolysis in comparison with the PBS group, whereas the H2O group skilled apparent hemolysis (Fig. 3H).
Collectively, these outcomes illustrated that personalized hydrogels introduced ECM-mimetic mesh construction with favorable swelling means and unparalleled biocompatibility, that are excellent candidates for masking, dressing substitute, and debridement of deep second diploma burn wounds.
Biocompatibility and chemokine sequestration of hydrogels. A, B, C) CCK-8 assay of HUVECs, NIH 3T3, and RAW264.7 cells co-cultured with PBS or hydrogels after 24 h (n = 3). D) Confocal microscopy photos of dwell/lifeless staining assay of Raw264.7 cells co-cultured with PBS or hydrogels after 24 h. Scale bar: 200 μm. E, F, G) MCP-1, VEGF, and IL-8 certain quantity to hydrogels. 100 nanograms of MCP-1, VEGF, and IL-8 was added to 50 µl totally different hydrogels and sequestration was decided after co-incubation with hydrogels for twenty-four h. H) Hemolysis images and ratio (%) after publicity to hydrogels. I) cumulative launch of PBNPs from PB@Algs-H hydrogels. (n = 3; imply ± s.d.; ns, not statistically vital; **p < 0.01, ***p < 0.001, ****p < 0.0001)
Anti-inflammatory results of Hydrogels in Vitro
Chemokines, ROS, and M1 macrophages are thought-about as main contributors of extreme wound irritation [12, 24, 30]. We designed an anti-inflammatory hydrogel impressed by biomolecular interactions to ameliorate the irregular inflammatory atmosphere in deep second-degree burn wounds, thereby resolving a sequence of scientific challenges concerning the remedy of inordinate inflammatory infiltration in wounds.
Algs Hydrogels Sequester chemokines
Many development components and chemokines, reminiscent of vascular endothelial development issue (VEGF), IL-8, and MCP-1, and so on. are termed heparin-binding proteins [17]. And their distribution in tissues is dominated by their complexation with sulfated GAGs of ECM or cell floor proteoglycans. Moreover, the binding affinity was discovered to rely upon the diploma of polysaccharide sulfation [17,18,19]. As such, we supplied an method to handle chemokine ranges in tissue to suppress excessive wound irritation based mostly on the interplay of GAGs with heparin-binding proteins. Three polysaccharides with totally different sulfation levels: Alg (0%), Algs-L (1%), and Algs-H (2%), have been synthesized by tuning the focus of HClSO3. Algs hydrogels have been examined for heparin-binding proteins seize by co-incubating with IL-8, MCP-1, and VEGF for twenty-four h (Fig. 3E-G). For IL-8, MCP-1, and VEGF, the Algs-H group adsorbed 71.3%, 70.5%, and 73.9%, respectively, and there was no pronounced distinction within the adsorption of the above three proteins between the PB@Algs-H and Algs-H teams, suggesting that the introduction of PBNPs didn’t have an effect on the seize properties of Algs-H. The Algs-L group scavenged 40.8%, 43.4%, and 32.8% of IL-8, MCP-1, and VEGF correspondingly, which was distinctly decrease than the PB@Algs-H and Algs-H teams, and the outcomes must be attributed to the variation within the variety of sulfated teams on Alg. Whereas the Alg and management teams appeared to have a specific amount of adsorption of those cytokines, there was no distinction between these two teams, indicating that the ultimate outcomes have been furnished by in vitro proteins degradation throughout incubation and there was no precise scavenging within the sense.
IL-8, a neutrophil chemokine, and MCP-1, a monocyte chemokine, are key mediators of irritation and are related to the long-term presence of immune cells in burn wound tissue. In distinction, VEGF takes a significant function within the reconstruction of broken tissues, and even determines the end result of wound restore. One of many explanation why the clinically favored hydrogel dressings that cowl fractured pores and skin and regulate moisture are much less efficient in deep second-degree burn wounds could also be that amplified protease ranges in wound tissue deplete pro-regenerative development components and speed up fibroblast senescence [14]. Nonetheless, our established hydrogel platform competently sequesters inflammatory chemokines and thus blocks the aggregation of inflammatory cells to the burn wound mattress whereas shielding the expansion components from protease degradation, which is undoubtedly an amazing profit to wound restore.
PB Nanozymes Scavenge ROS and alleviate intracellular oxidative stress
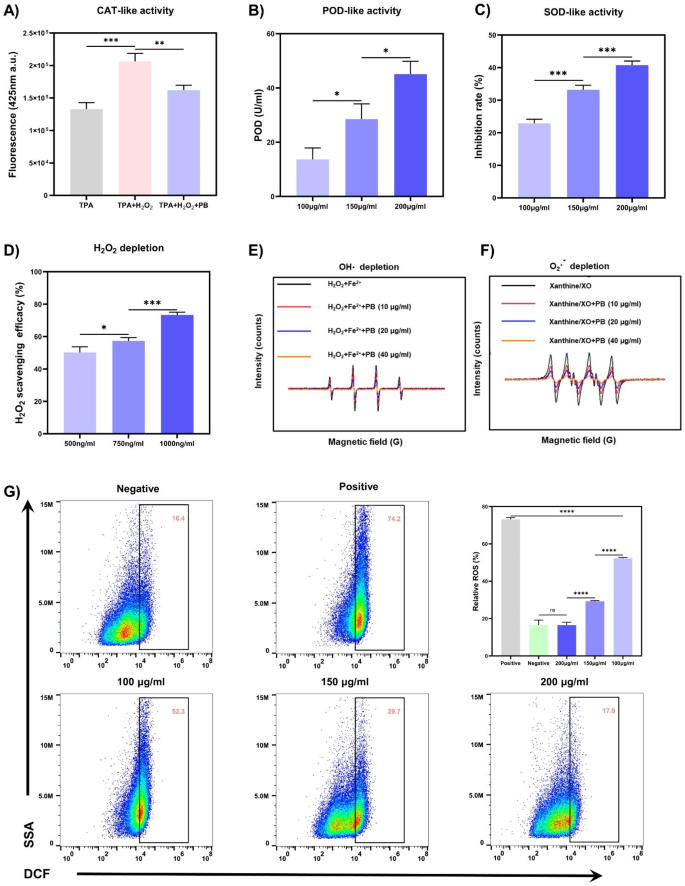
ROS have been acknowledged as pivotal mediators within the initiation and development of wound irritation [29]. PB nanozymes afford cells safety in opposition to ROS-induced oxidative stress by scavenging varied ROS together with O2·−, OH·, and H2O2 [37]. The capability of PB nanozymes to scavenge H2O2 was initially explored by using the response of titanium sulfate with H2O2 to generate a yellow titanium peroxide-titanium advanced with a attribute peak at 415 nm. After PB nanozymes have been added to the response system, the H2O2 was consumed and the absorbance at 415 nm decreased, and the clearance of H2O2 may very well be calculated. As seen in Fig. 4D, the H2O2 consumption by PB nanozymes was concentration-dependent, with a 73.3% H2O2 scavenging fee at a PBNPs focus of 1000 ng/ml. CAT or CAT-mimics catalyze the decomposition of H2O2 to yield O2 and H2O, defending the physique from oxidative injury induced by H2O2. To additional elucidate the CAT mimetic exercise of PBNPs, the catabolism of H2O2 was assessed with disodium terephthalate (TPA-Na), and the non-fluorescent TPA-Na might rework H2O2 right into a fluorescent indicator with a attribute peak at 425 nm. In Fig. 4A, the fluorescence depth at 425 nm decreased sharply within the presence of PBNPs, implying their competent CAT-like efficiency. Subsequent, we evaluated the OH· depletion capability of PBNPs by electron paramagnetic resonance (EPR) with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin probe (Fig. 4E). The OH· is initially generated by the Fe2+/H2O2 response system with a definite OH· sign within the EPR. And the OH· sign depth dramatically declined in a concentration-dependent method with the addition of PBNPs, testifying that the OH· sign was considerably attenuated after low-dose PBNPs disposal. POD can be an antioxidant enzyme that detoxifies H2O2 into water and might catalyze the oxidation of guaiacol to provide a tea-brown substance within the presence of H2O2, which has a most absorption at 470 nm. After including totally different concentrations of PBNPs to the response system, the POD-like exercise of PBNPs was measured by detecting the absorbance at 470 nm. And the best POD-like exercise of 45.1 U/ml was noticed at 200 µg/ml of PBNPs (Fig. 4B). Lastly, SOD is one other classical antioxidant enzyme that turns O2·− into H2O2 and O2, and an SOD assay package was operated to quantify the SOD mimetic exercise of PBNPs. The O2·− was generated by the xanthine (X)/xanthine oxidase (XO) system, which convert the WST-1 into WST-1 formazan with an absorption peak at 450 nm (Fig. 4C). The SOD-like exercise of PBNPs was additionally concentration-dependent, with the utmost SOD exercise of 40.8% at 200 µg/ml of PBNPs. Taking DMPO because the spin probe, we additional confirmed the flexibility of PBNPs to scavenge O2·− by EPR. Within the X/XO system, there appeared an intense attribute sign peak of DMPO/O2·−. After the addition of PBNPs (10 µg/ml), the sign depth dropped clearly, and when the focus of PBNPs was additional elevated to 40 µg/ml, the sign depth decreased steeply, indicating that PBNPs possessed superior scavenging means for O2·−.
As well as, to evaluate the protecting impact of PBNPs on cells in opposition to oxidative stress, 2′,7′-Dichlorofluorescin diacetate (DCFH-DA) was used to detect ROS ranges in HaCaT cells. After cells have been uncovered to 750 µM H2O2, the intracellular ROS stage within the optimistic management group raised to 73.13%, which was remarkably larger than that within the unfavorable management group, and intracellular ROS ranges have been significantly decrease within the pretreatment group with totally different concentrations of PBNPs in comparison with the optimistic management group. Importantly, the intracellular ROS stage within the pretreatment group with 200 µg/ml of PBNPs was approached to that within the unfavorable management group, signifying that 200 µg/ml of PBNPs might successfully keep away from H2O2-induced elevation of intracellular ROS ranges and keep intracellular ROS at a traditional stage. Due to this fact, PBNPs with distinguished means to scavenge ROS are auspicious to reshape the tough ROS microenvironment and function a promising antioxidant in deep second-degree burn wounds.
Algs Hydrogels regulate the polarization of macrophages
Macrophages carry out a number of crucial capabilities all through the wound therapeutic course of, recognizing and eradicating pathogens, mobile particles, and phagocytosing apoptotic neutrophils within the early levels, and forwarding angiogenesis, collagen deposition, and epithelial cell migration within the later phases [24,25,26]. Sadly, the phenotypic conversion of pro-inflammatory M1 macrophages right into a pro-resolution state tends to be dysregulated in deep second-degree burn wounds, accounting for a poor regenerative atmosphere [28]. Just lately, it has been demonstrated that sulfated GAGs can induce the polarization of macrophages from M1 to M2 [53]. Sulfated GAGs with extremely negatively charged teams can modulate the distribution of some heparin-binding proteins throughout the ECM [17,18,19], which, in flip, eases the horrible inflammatory atmosphere round macrophages. Moreover, some distinctive sulfated GAGs with pentasaccharide sequence, reminiscent of sulfated chitosan, instantly work together with macrophage floor receptors, thus activating pathways related to macrophage polarization [53, 54]. Due to this fact, we speculate that Algs sharing related construction with sulfated chitosans, may set off the macrophages transition from M1 to M2.
A number of enzyme-like actions and cytoprotection in opposition to ROS of PBNPs. (A) CAT-like exercise of PBNPs decided by TPA-Na. (B) POD-like exercise of PBNPs using guaiacol because the chromogenic substrate. (C) SOD-like exercise of PBNPs decided by an SOD assay package. (D) H2O2 depletion of PBNPs decided by titanium sulfate methodology. (E) OH· depletion capability of PBNPs decided by EPR. (F) O2·− depletion of PBNPs decided by EPR. (G) ROS in HaCaT cells was detected by stream cytometry mixed with DCFH/DCF ROS detecting system. (n = 3; imply ± s.d.; ns, not statistically vital; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
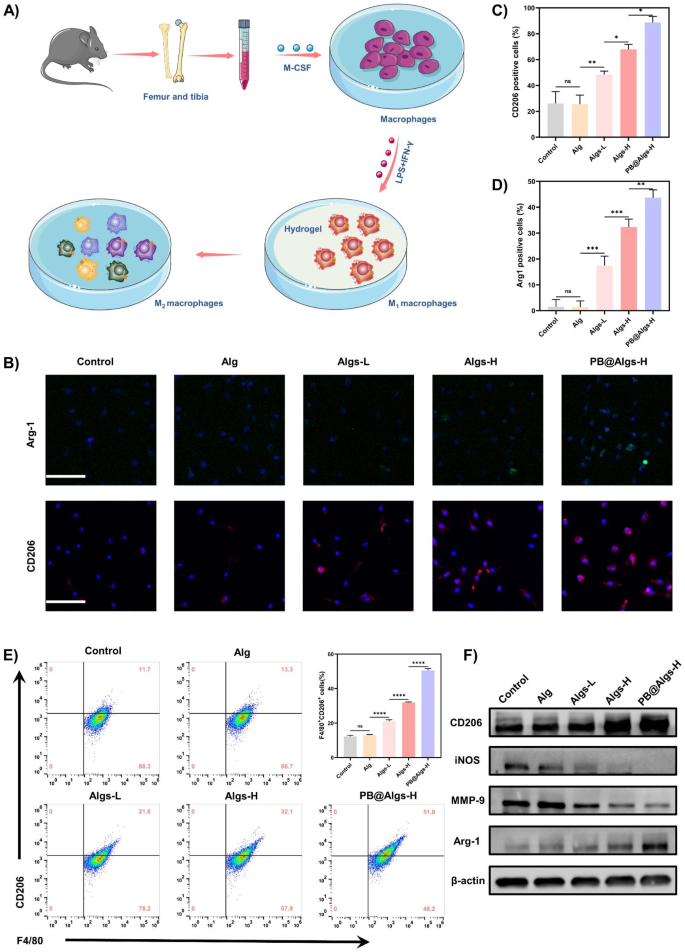
To confirm this speculation, F4/80 and CD11b double-positive macrophages of bone marrow origin have been first extracted, purified, and characterised by stream cytometry (Fig. 5A). Subsequent, lipopolysaccharide (LPS) and interferon-γ (IFN-γ) have been employed to stimulate bone marrow-derived macrophages to polarize towards M1 (Determine S3A). After finishing these pre-processes, we analyzed the consequences of hydrogels on M1 macrophages by immunofluorescence. M1 macrophages have been induced to considerably categorical CD206 and Arg-1 related to the M2 macrophages within the cell membrane and cytoplasm after co-incubation with Algs-L, Algs-H, and PB@Algs-H hydrogels (Fig. 5B-D). Amongst them, the PB@Algs-H group obtained the utmost expression of CD206 and Arg-1, whereas the fluorescence depth of the management and Alg teams have been weak and there was no obvious distinction between them. We quantified the proportion of M2 macrophages (F4/80+, CD206+) after stimulation with hydrogels by stream cytometry in parallel. The PB@Algs-H group acquired the most important proportion of M2 macrophages, in settlement with the immunofluorescence outcomes (Fig. 5E). Then, we quantitatively analyzed the relative mRNA expression of M1 macrophage marker genes (iNos, TNF-α, IL-6, and Il-1β) and M2 macrophages marker genes (Arg1, Il-10, and VEGF) by RT-qPCR (Determine S3F and G). The outcomes of RT-qPCR remained in keeping with earlier findings. Lastly, the expression of M1 macrophages markers (iNOS and MMP-9) and M2 macrophages markers (CD206 and Arg-1) have been detected by western blot. The relative expression of M1 macrophages markers was considerably decrease within the Algs-L, Algs-H, and PB@Algs-H teams in comparison with the management and Alg teams, whereas the relative expression of M2 macrophage-related markers was markedly larger (Fig. 5F and Determine S3B-E), which was coherent with the outcomes of immunofluorescence and stream cytometry. Clearly, Alg didn’t induce the phenotypic conversion of M1 macrophages, whereas Alg owned such a property after being sulfated, authenticating our conjecture that the introduction of the sulfate group granted it with this operate. The truth is, the above outcomes additionally uncovered that the PB@Algs-H group possessed stronger potential to facilitate the polarization of M1 macrophages in comparison with the Algs-H group. This can be associated to the truth that PB nanozymes in PB@Algs-H hydrogels scavenged ROS within the tradition atmosphere and inhibited the stimulation on macrophages from ROS, which has been reported within the literature. The discount of M1 macrophage-derived inflammatory mediators, reminiscent of tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), and proteases (e.g., MMP-9) will modulate the wound microenvironment, attenuate tissue injury, and enhance development indicators. In distinction, the ornithine from M2 macrophage-associated Arg-1 can promote cell proliferation and VEGF secretion, which can bolster neovascularization, thereby elevating the potential for tissue regeneration and restoration.
Macrophage polarization in response to Algs hydrogels in vitro. (A) Illustration of in vitro bone marrow-derived macrophage (BMDMs) stimulation assays. (B) Consultant immunofluorescence staining photos of CD206 and Arg-1 (M2 macrophages marker) of BMDMs. Scale bar: 100 μm. C, D) Statistical information of the proportion of Arg-1+ and CD206+ macrophages. E) Circulate cytometry evaluation indicating the proportion of M2 macrophages (F4/80+CD206+). F) Protein expression stage of CD206, iNOS, MMP-9, and Arg-1 in macrophages decided by western blot. (n = 3; imply ± s.d.; ns, not statistically vital; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
Results of the hydrogels on Deep Second-Diploma burn wounds in vivo
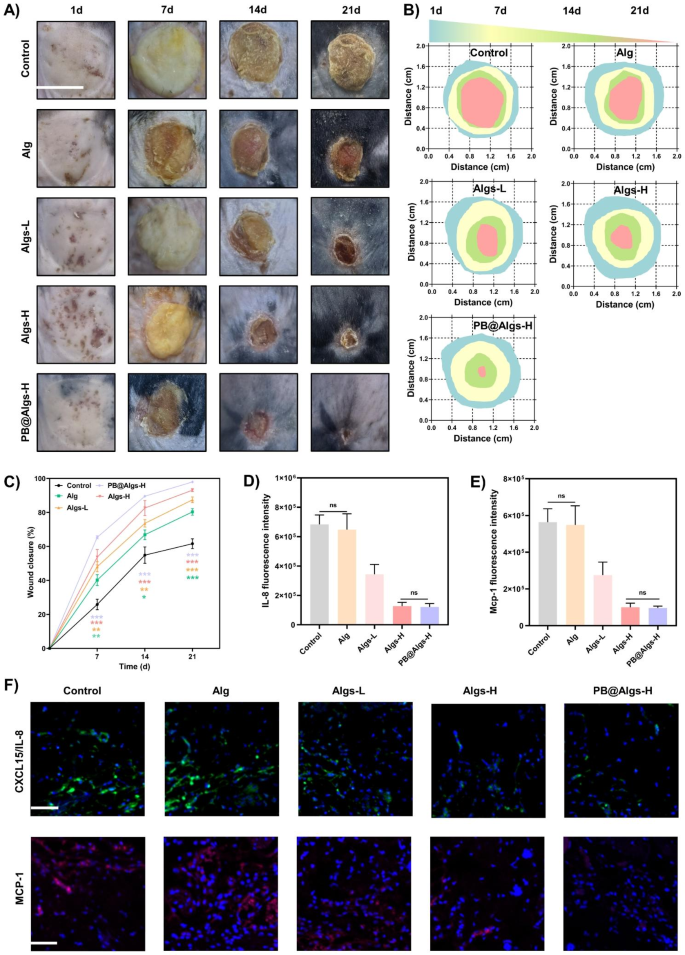
In vitro research have confirmed that Algs hydrogels possessed highly effective anti-inflammatory capability. Due to this fact, a deep second-degree burn mannequin in mice was made to guage the therapeutic results of hydrogels. To amass correct burn space and depth, an electrical scalding instrument with managed temperature and strain was utilized for modeling. In contrast with the conventional pores and skin tissue of mice, the entire dermis and deep dermis of the experimental group have been devastated, and solely the deep pores and skin attachment remained, however the subcutaneous fats layer and muscle tissue have been structurally intact and undamaged. Importantly, the burn diploma remained constant throughout experimental teams, minimizing its influences on the ultimate outcomes (Determine S4A). Subsequent, deep second-degree burn wounds in mice have been handled with saline dressing (management group), Alg, Algs-L, Algs-H, and PB@Algs-H hydrogels and assessed by remark of therapeutic fee and tissue assortment on days 1, 7, 14, and 21. Macroscopically, 4 hydrogel-treated teams (Alg, Algs-L, Algs-H, and PB@Algs-H) healed sooner compared with the management group, evidencing the therapeutic impact of hydrogels. Amongst them, the PB@Algs-H group attained the best therapeutic fee, with 65%, 90%, and 98% therapeutic ratio on days 7, 14, and 21, respectively, and solely 26%, 55%, and 62% wound closure within the management group (Fig. 6B and C). The management group acquired nearly invalid remedy, and the deplorable wound irritation should be resolved by itself, which is a tough and long-term course of. Curiously, in vitro research have disclosed that Alg is nearly extraneous to anti-inflammation, however the Alg group had a sooner therapeutic fee than the management group. The consequence could in all probability attribute to the truth that the hydrogels vigorously absorbed the exudate, induced autolysis of necrotic tissue, and accelerated wound mattress autodebridement, which is noteworthy to alleviate wound irritation. The persistence of necrotic tissue on the wound mattress will recruit a relentless stream of inflammatory cells, aggravating the wound irritation. Consequently, this may increasingly even be one of many causes for the extensive utility of Alg hydrogels in scientific burn wounds.
Results of the hydrogels on selling deep second-degree burn wound therapeutic in mice. (A) Consultant photos of wound therapeutic course of in deep second-degree burn wound mice. Scale bar: 1 cm. (B) Schematic diagram of the wound therapeutic handled with hydrogels throughout 21 days. (C) Quantitative information of relative wound closure at totally different time factors. D, E) Statistical information of IL-8/CXCL15 and MCP-1 fluorescence depth. F) Consultant IL-8/CXCL15 and MCP-1 immunofluorescence staining photos on day 2 after remedy with hydrogels. Scale bar: 100 μm. (n = 3; imply ± s.d.; ns, not statistically vital; *p < 0.05, **p < 0.01, ***p < 0.001)
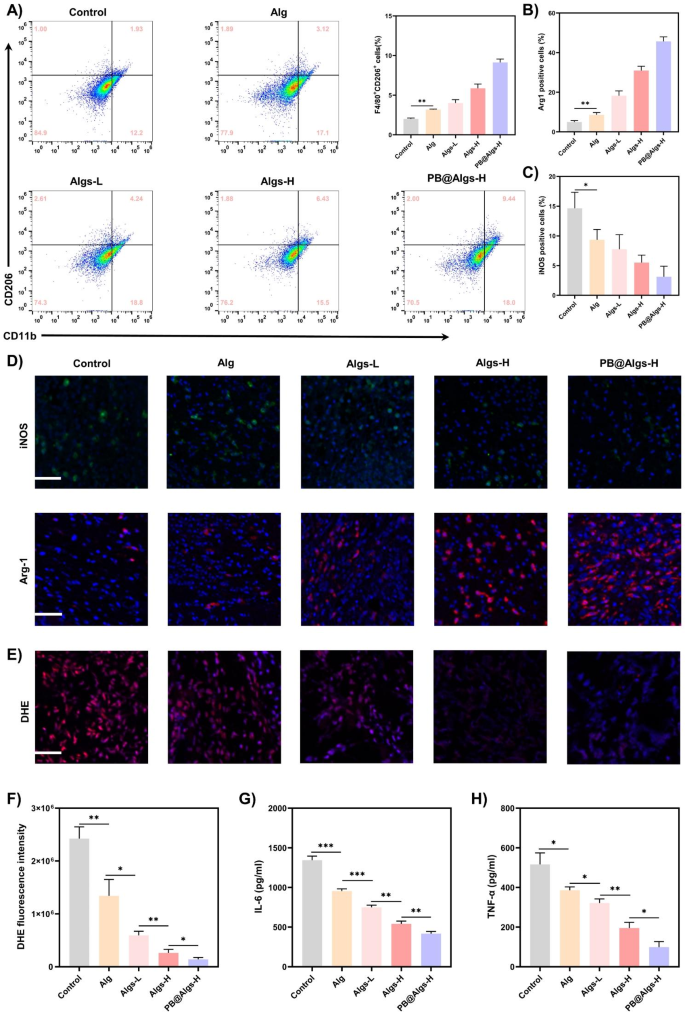
Wound therapeutic is an orchestrated cascade with 4 overlapped however distinct phases: hemostasis, irritation, proliferation, and reworking [55]. Any endogenous and exogenous adversarial components can disrupt the physiological therapeutic course of, of which the inflammatory section is essentially the most inclined to disturbance [56, 57]. The deep second-degree burn wounds are characterised by considerable inflammatory cells infiltration and a excessive stage of pro-inflammatory cytokines within the early levels because of extreme tissue injury. Sadly, the terrible inflammatory atmosphere precludes the transition from the inflammatory to the proliferative section of the wound therapeutic [4,5,6]. On this context, we evaluated the impression of hydrogels on the inflammatory section of wound restore. We first analyzed the IL-8/CXCL15 and MCP-1 adsorption by hydrogels utilizing immunofluorescence on day 2 (IL-8 is just not current in mice and CXCL15 is its corresponding mouse homologue). There was no apparent distinction within the presence of those two chemokines between Alg and the management group, each of which shared a large prevalence. Whereas the fluorescence depth of those two crucial inflammatory mediators was drastically declined within the Algs-H and PB@Algs-H teams in comparison with the Alg and management teams, and there was no seen distinction between these two teams, which was in keeping with in vitro outcomes (Fig. 6D-F). The persistence of quite a few IL-8/CXCL15 and MCP-1 within the wound will appeal to extra inflammatory cells reminiscent of neutrophils and monocytes, initiating a vicious cycle of wound irritation. Subsequent, on day 4, we measured the expression of two different key inflammatory components, TNF-α and IL-6, by ELISA. As a secondary impact of the repressed accumulation of immune cells within the wound, the TNF-α and IL-6 expression in Algs-contained teams was significantly decreased relative to the management group and Alg teams, particularly within the PB@Algs-H group, wherein PB nanozymes eliminated substantial ROS apart from the chemokine sequestering impact of Algs-H (Fig. 7G and H). On prime of that, we discovered that there was a downregulation of TNF-α and IL-6 expression within the Alg group over the management group, which is aligned with the noticed outcomes of wound therapeutic. Whereas Alg seems to be irrelevant to the decision of irritation, their function in accelerating the method of autolysis and inducing autolytic wound cleaning is reconfirmed. Then, the in vivo antioxidant capability of PB nanozymes was validated by using a ROS-sensitive dihydroethidium (DHE) probe to detect ROS ranges in wound tissue. As compared with the management group, the DHE fluorescence depth was evidently depressed within the hydrogel teams, amongst which the PB@Algs-H group mirrored the weakest fluorescence depth, completely testifying to the antioxidant exercise of PB nanozymes. though Alg or Algs itself lacked evident antioxidant exercise, the DHE fluorescence depth of the Alg, Algs-L, and Algs-H teams weakened sequentially. The inflammatory microenvironment in deep second-degree burn wounds is extraordinarily advanced, which varies as Alg or Algs hydrogels progressively train their distinctive therapeutic results on the wound, thus affecting the manufacturing and quenching of ROS. This may increasingly account for the recognition of in depth practical hydrogels. The inflammatory section of deep second-degree burn wounds is noticeably extended, which can be related to the failure of phenotype swap of macrophages well timed. In vitro research have already evidenced the potential of Algs to induce the polarization of M1 macrophages towards M2-like macrophages. The impact of hydrogels on M1 macrophages in deep second-degree burn wounds was additional investigated. On day 7, the junction of the inflammatory and proliferative phases, wound tissue was collected and ready as single cell suspensions for stream cytometry to quantify the proportion of M2 macrophages. The proportion of M2 macrophages (CD11b+, CD206+) was distinctly larger within the Algs-L, Algs-H, and PB@Algs-H teams than that within the untreated group, which remained in keeping with the in vitro outcomes (Fig. 7A and S3H). Curiously, we discovered that the Alg group owned a better share of M2 macrophages compared to the management group, which was not corresponding with the in vitro outcomes. This may increasingly nonetheless be pertinent to the therapeutic results of Alg hydrogels we mentioned beforehand, and the alteration of the inflammatory wound atmosphere introduced a phenotype shift of macrophages. Subsequent, the impacts of hydrogels on M1 macrophages have been additional evaluated by immunofluorescence staining on M1 macrophages (iNOS) and M2 macrophages (Arg-1). The outcomes confirmed the developments noticed in up to now, with the PB@Algs-H group reaching the best M2/M1 macrophage ratio of all teams (Fig. 7B-D). On day 7, the density of M1 macrophages was decrease within the hydrogel-treated group, whereas the density of M2 macrophages was comparatively larger than that within the management group, indicating that the wound therapeutic course of was steadily progressing into the proliferative section. In distinction, loads of iNOS+ macrophages have been nonetheless seen within the management group, denoting that the inflammatory section was nonetheless ongoing. With the well timed phenotype transformation of M1 macrophages, the multifunctional hydrogels additional curbed the inflammatory response in wound therapeutic, which, in flip, boosted angiogenesis and tissue reworking. Accordingly, the PB@Algs-H hydrogels put exceptional anti-inflammatory results on deep second-degree burn wounds and accelerated the transition from the inflammatory section to the proliferative stage, subsequently encouraging tissue regeneration.
Anti-inflammatory results of hydrogels on deep second-degree burn wounds. A) Circulate cytometry evaluation indicating the proportion of M2 macrophages (CD11b+CD206+) on day 7. B, C) Statistical information of the proportion of Arg-1+ and iNOS+ macrophages. D) Consultant immunofluorescence staining photos of iNOS and Arg-1 on day 7. Scale bar: 100 μm. E) DHE staining photos indicating ROS ranges in wound tissue on day 7. Scale bar: 100 μm. G, H) Concentrations of TNF-α and IL-6 in wound tissue measured by ELISA on day 4. (n = 3; imply ± s.d.; *p < 0.05, **p < 0.01, ***p < 0.001)
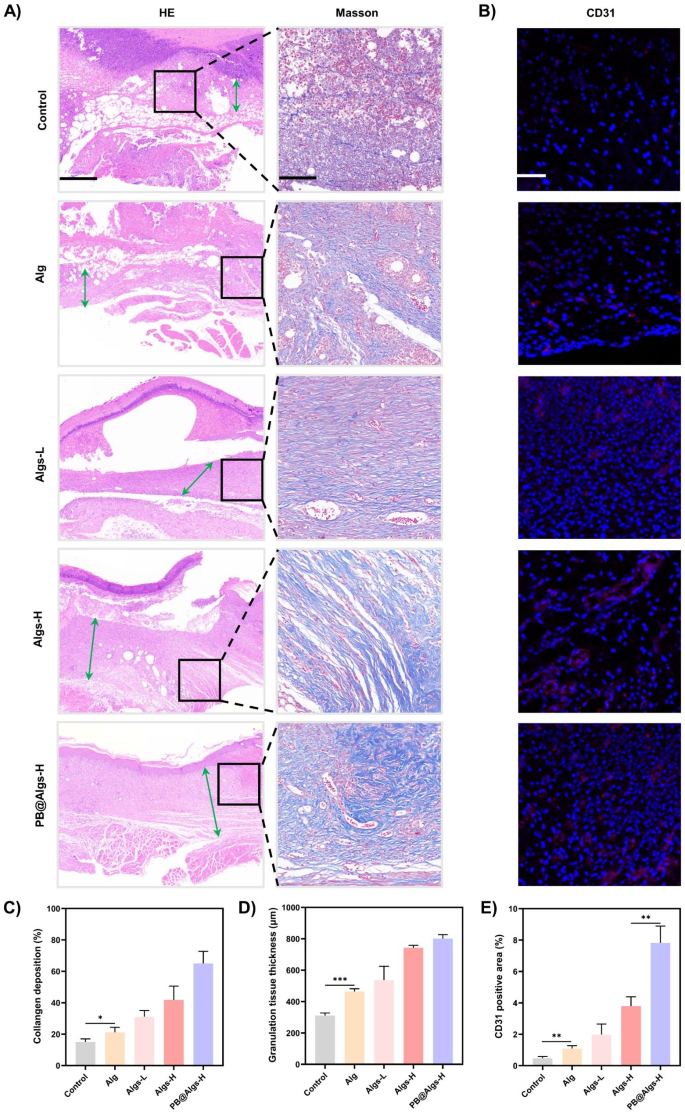
Subsequent, we assessed the influences of hydrogels on wound tissue regeneration and reworking. In the course of the proliferative section, neovascularization is crucial to make sure enough nutrient and oxygen supply to the wound tissue to assist fibroblast proliferation, collagen synthesis, and re-epithelialization [58]. Immunostaining for platelet endothelial cell adhesion molecule-1 (CD31) was carried out on day 14 to examine the presence of neovascularization within the granulation tissue. The CD31 expression was higher in hydrogel teams than that within the management group, with the best expression stage in PB@Algs-H group, which is line with with the therapeutic outcomes noticed below macroscopy (Fig. 8B and E). Within the reworking section, correct collagen deposition and reworking is important to reinforce the tensile energy of the tissue and assist wound therapeutic. The formation of recent collagen was noticed by Masson’s trichrome staining, and the thickness of granulation tissue in H&E staining was examined synchronously. On day 21, profuse residual dry scabs might nonetheless be observable within the management group, implying a delayed therapeutic of deep second-degree wounds (Fig. 8A). Quite the opposite, the hydrogel-treated wounds exhibited a thicker granulation layer and extra collagen deposition (Fig. 8C and D). Amongst them, the PB@Algs-H group displayed the best thickness of granulation tissue and the widest vary of collagen deposition, disclosing that the broken tissues within the PB@Algs-H group have been near restoration and maturation and approaching regular pores and skin. The favorable efficiency of the proliferative and reworking phases in hydrogel teams was attributed to the truth that Algs and PB nanozymes tremendously restricted the headway of disproportionate irritation within the early levels and expedited the development of the wound therapeutic from the inflammatory to the proliferative section.
Results of anti-inflammatory hydrogels within the proliferative and reworking phases of wound therapeutic. (A) HE staining of the wound tissue mirrored the thickness granulation tissue (inexperienced arrows) on day 21. Scale bar: 500 μm. Masson’s trichrome staining of the wound tissue mirrored collagen deposition space on day 21. Scale bar: 100 μm. (B) Consultant immunofluorescence staining photos of CD31 mirrored neovascularization. Scale bar: 100 μm. (C) Statistical information of collagen deposition space. (D) Statistical information of granulation tissue thickness. (E) Statistical information of CD31 optimistic space within the wound tissue. (n = 3; imply ± s.d.; *p < 0.05, **p < 0.01, ***p < 0.001)
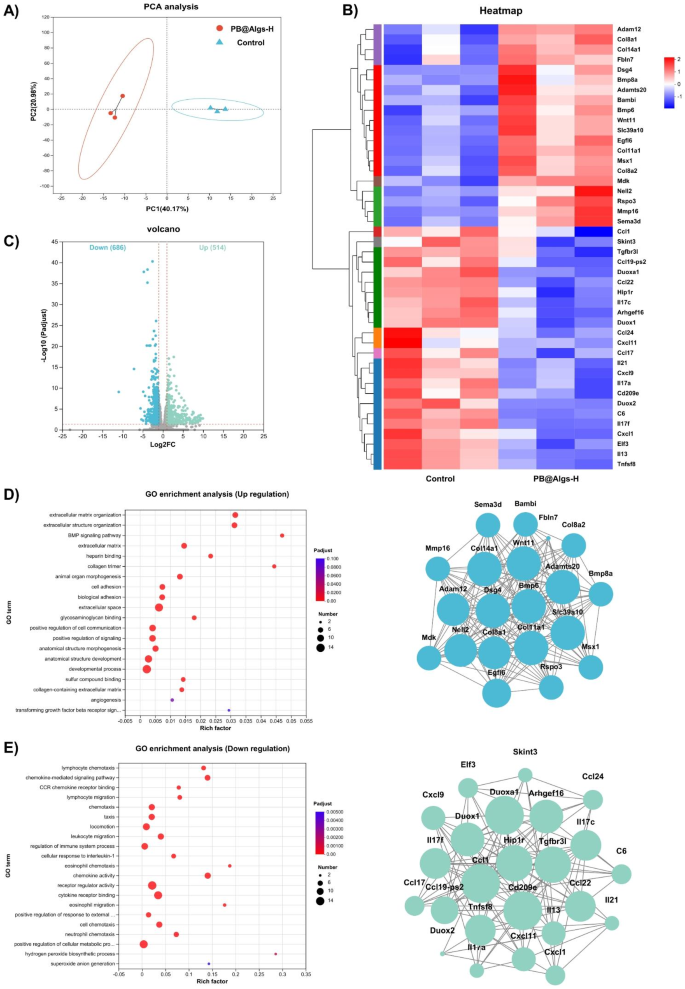
To comprehensively consider the wound microenvironment after remedy with PB@Algs-H hydrogels, RNA sequencing was carried out on wound tissue on day 7 in line with the in vivo experiment outcomes, and the untreated deep second-degree burn wound tissue was taken as a management. The unguided principal element evaluation (PCA) confirmed vital variations in transcriptomic profiles between the management and PB@Algs-H teams (Fig. 9A). Based on the empirical Bayes methodology, 1200 considerably differentially expressed genes (DEGs) have been recognized after administration of PB@Algs-H hydrogels, of which 514 have been upregulated and 686 have been downregulated, as proven within the volcano plot (Fig. 9C). Then, DEGS-based enrichment evaluation was performed on the up- and down-regulated gene units. Hierarchical clustering evaluation screened and separated for variations in gene expression between the management and PB@Algs-H teams of mouse wound tissue (Fig. 9B). Notably, the expression of genes concerned in wound irritation (Ccl24, Ccl1, Il17a, Cxcl1, Cxcl9, Ccl22, Ccl17, and Cxcl11) was considerably downregulated after PB@Algs-H remedy, as have been genes associated to ROS manufacturing (Duox1, Duox2, and Duoxa1). Nonetheless, genes related to tissue regeneration and proliferation (Egfl6, Col14a1, Adamts20, Col11a1, Mmp16, Col8a2, and Col8a1), and genes concerned in heparin protein binding (Rspo3, Nell2, Mdk, Fbln7, and Col11a1) have been considerably upregulated. Gene ontology (GO) evaluation uncovered that the upregulated genes have been primarily targeted on extracellular matrix synthesis and heparin protein binding (Fig. 9D), whereas the downregulated genes have been principally related to inflammatory cell migration and oxidative stress (Fig. 9E). The outcomes revealed that the aggregation of inflammatory cells to the wound mattress was promptly interrupted and the superfluous synthesis of ROS was restrained in handled deep second-degree wounds. And the excessive inflammatory infiltration of the burn wounds was relieved, whereas exhibiting glorious regenerative potential and optimistic response to development indicators.
Subsequent, up and down-regulation datasets have been collected for Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment evaluation. KEGG pathway enrichment evaluation revealed that PB@Algs-H hydrogels potently suppressed some inflammatory pathways reminiscent of chemokine signaling pathway and IL-17 signaling pathway (Determine S5A), which have been correlated with progressive wound irritation. Inhibition of those pathways confirmed the anti-inflammatory results of PB@Algs-H hydrogels in deep second-degree wounds. Moreover, The KEGG of up-regulation dataset was targeted on TGF-β signaling pathway and Wnt signaling pathway (Determine S5B), which have been related to cell migration, proliferation, and ECM reconstruction. Dynamic crosstalk within the WNT and TGF-β signaling pathways would possibly tremendously advance the wound therapeutic development. The mitigation of hyperinflammation and activation of development signaling would possibly ship an improved therapeutic consequence. To confirm the RNAseq outcomes, we quantitatively analyzed key genes in these pathways described above, and the RNA relative expression ranges have been in keeping with KEGG outcomes (Determine S5C and D).
Complete assessments of the wound microenvironment after remedy with PB@Algs-H hydrogels utilizing RNA sequencing. (A) PCA based mostly on DEGs within the wound tissue between the management and PB@Algs-H teams. (B) Warmth map of upregulated and downregulated genes within the deep second-degree burn wound microenvironment after remedy with PB@Algs-H hydrogels (fold change ≥ 4 and P < 0.05). (C) Volcano plots displaying the upregulated and downregulated genes in response to PB@Algs-H hydrogels remedy. (D) GO enrichment evaluation of the upregulated genes. (E) GO enrichment evaluation of the downregulated genes
[ad_2]