[ad_1]
Preparation and characterisation of FMA NPs
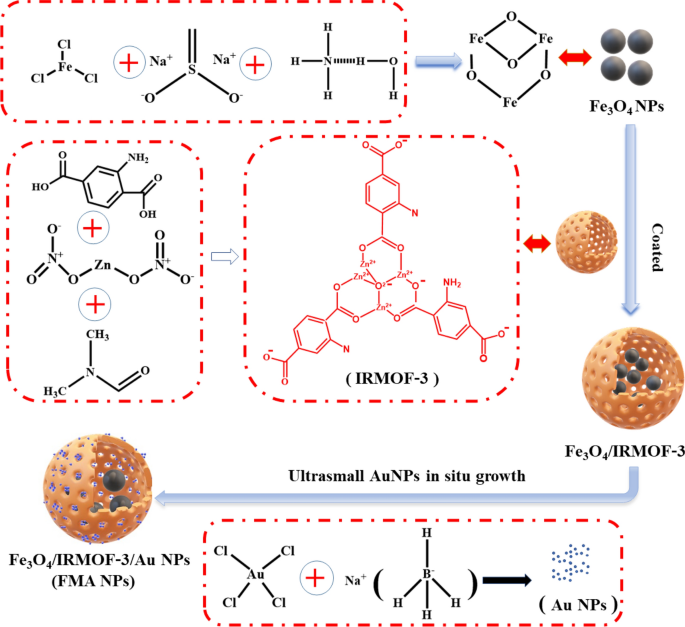
We developed an modern nanocatalytic antibacterial nanosystem utilizing IRMOF-3-modulated biomimetic hybrid FMA NPs. The FMA NPs had been assembled with ultrasmall Au NPs and Fe3O4 NPs displaying synergistic POD-mimicking actions for contaminated wound therapeutic with out utilizing any antibiotics. Scheme 2 presents the artificial mechanisms of hybrid FMA nanozymes: (i) preparation of superparamagnetic Fe3O4 NPs by way of partial discount chemical co-precipitation; (ii) modification of Fe3O4 NPs with extremely porous IRMOF-3 shells to manufacture Fe3O4@MOF NPs utilizing the hydrothermal technique; and (iii) incorporating ultrasmall Au NPs into the pores of IRMOF-3 shells in situ, which enhanced their POD actions by way of a cascade response to type versatile multiphase catalysts FMA NPs.
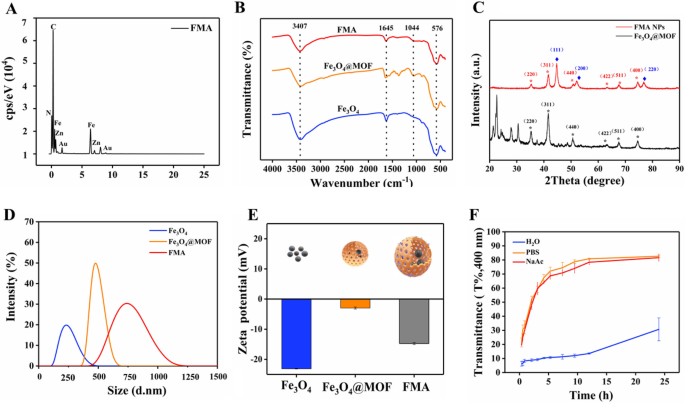
The as ready nanocomposites had been characterised as follows. The morphological patterns of the ready Fe3O4 NPs, Fe3O4@MOF NPs and FMA NPs had been characterised by transmission electron microscopy (TEM). TEM picture reveals a spherical or elliptic construction of Fe3O4 NPs with a diameter of round 10 nm (Extra file 1: Fig. S1A). The Fe3O4@MOF NPs confirmed uniform spherical or extra common squares (Extra file 1: Fig. S1B). The schematic diagram of FMA NPs is proven in Fig. 1A. As proven in Fig. 1B and C, the TEM photos of FMA NPs show a core-shell construction with a median particle dimension about 30 nm. The ultrasmall and dispersed AuNPs are successfully wrapped into pore channels of IRMOF-3 shells, thus bettering their catalytic efficiency. Elemental mapping evaluation was used to precisely assess the fundamental composition of the catalytic FMA NPs. The outcomes counsel that Au Fe, O, and Zn co-exist with FMA NPs within the vitality spectrum vary (Fig. 1D-I). The uniform distribution of Au in FMA NPs was verified by EDS elemental mapping (Fig. 2A).
The composition of the fabricated numerous nanocomposites was analysed by way of Fourier-transform infrared spectroscopy (FTIR). The attribute Fe-O-Fe spectral band appeared at 576 cm−1 as a result of IRMOF-3-coated Fe3O4 NPs. The absorption peak of C-O single bond was detected at 1044 cm−1. The N-H stretching vibration peak at 3407 cm−1 corresponds to the everyday amino purposeful group of IRMOF-3, indicating profitable coating of IRMOF-3 on Fe3O4 NPs (Fig. 2B). Following anchoring of Au NPs into the pore area, a definite absorption peak C-N of Fe3O4@MOF NPs appeared at 1645 cm−1, indicating intact chemical construction of IRMOF-3-modified Fe3O4 NPs. The outcomes instructed profitable fabrication of FMA NPs as anticipated.
To exactly analyse the composition of Fe3O4@MOF and FMA NPs, the crystal buildings of the complexes had been analysed by way of X-ray diffraction (XRD). The diffraction peaks at 2θ = 35.2°, 41.5°, 50.6°, 63.0°, 67.3°, and 74.2° correspond to the (220), (311), (400), (422), (511), and (440) crystal planes, respectively, of the Fe3O4 NPs (JCPDS 65-3107) (Fig. 2C). The diffraction peaks of MOF match the X-ray diffraction spectra reported in related research beforehand [46, 47], indicating that the formation of the MOF shell layer didn’t change the crystal construction of the Fe3O4 NP. After the in situ formation of Au NPs no MOF peaks had been detected within the core-shell construction of the ultimate service. The crystallinity of MOF particles will depend on the solvent stage within the interior pores [48, 49]. Subsequently, heating of the thinner MOF shell layer in the course of the synthesis induces amorphous transition of the crystalline MOF shell layer. Nevertheless, the FMA NPs 2θ = 44.7°, 52.1°, and 76.8° strongly correspond to the crystalline planes of (111), (200), (220) of the usual card of Au NPs (JCPDS 04-0784), respectively. They’re additionally strongly in step with the attribute diffraction peaks of Fe3O4 NPs, suggesting that Au NPs are strongly certain to the IRMOF-3 shells. These outcomes display the profitable fabrication of FMA NPs.
The hydration particle dimension distribution of varied nanoparticles was analysed with a dynamic laser scattering (DLS) in DI water. As proven in Fig. 2D, Fe3O4 NPs exhibit a broad particle dimension distribution with a median hydration particle of 320 nm, whereas the common hydration particle of Fe3O4@MOF NPs is 450 nm. Loading of Au NPs will increase the hydration particle dimension of FMA NPs to about 600 nm. The hydration particle dimension of Fe3O4 NPs, Fe3O4@MOF NPs, and FMA NPs attended to extend sequentially, indicating profitable coating of the varied modified layers. The evaluation of zeta potential confirmed that the fees of Fe3O4, Fe3O4@MOF, and FMA NPs had been roughly − 23.1, -2.97 and − 14.7, respectively (Fig. 2E). The general outcomes point out the profitable modification of various coating layers. As well as, the soundness of FMA NPs in several media at completely different time factors was investigated. As proven in Fig. 2F, the FMA NPs confirmed the most effective stability in numerous media after 24 h therapy in DI. The soundness of FMA NPs in PBS buffer was the worst, which can be attributed to the presence of ample salt ions within the buffer. Neutralisation of the floor cost of the nanocomposites resulted in aggregation or precipitation of enormous particles. The soundness of FMA NPs in NaAc-HAc buffer resolution was intermediate. Nevertheless, it was wonderful after 10 h of experiment. Subsequently, the NaAc-HAc resolution was chosen for additional experiments.
Characterisation of various nanocomposites. A Distribution of chemical parts of FMA NPs. B FTIR spectrum of Fe3O4, Fe3O4@MOF, and FMA NPs. C XRD patterns of Fe3O4@MOF and FMA NPs. D Measurement distribution of hydrated particles. E Zeta potential assay of the ready Fe3O4, Fe3O4@MOF, and FMA NPs. F Evaluation of stability of FMA NPs in numerous media together with DI water, PBS resolution (0.01 mol L−1, pH 7.4), and NaAc-HAc buffer (0.02 mol L−1, pH 3.6) by DLS
Analysis of intrinsic POD-mimicking actions of FMA NPs and detection of ·OH technology
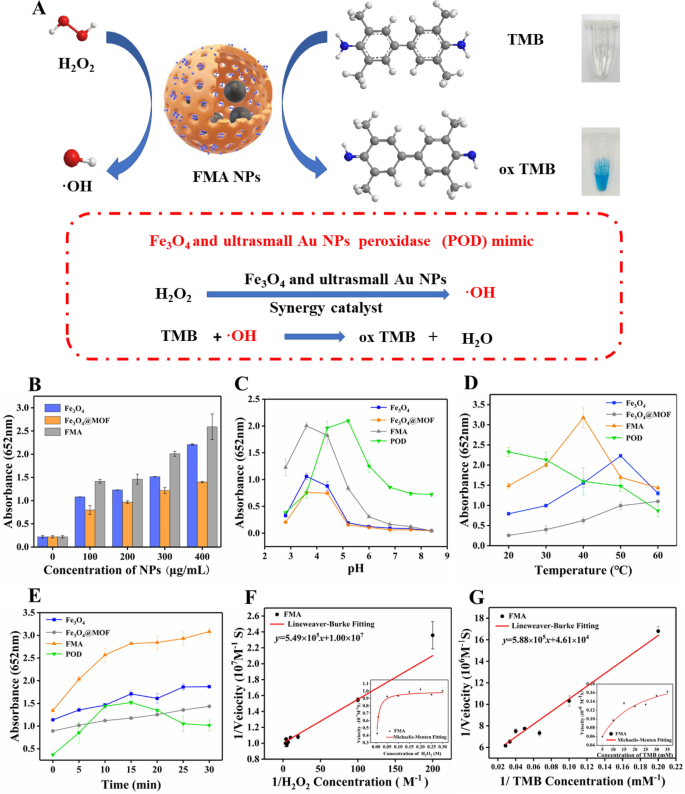
Determine 3 A illustrates the catalytic mechanism of FMA NPs. The FMA NPs cleaved H2O2 into•OH radicals. The mix of Fe3O4 NPs and ultrasmall Au NPs displayed a synergistic POD-like exercise. The colourless TMB was oxidised within the presence of •OH, leading to a blue coloration. In contrast with the catalytic exercise of single Fe3O4 NPs or ultrasmall Au NPs, the addition of Au NPs considerably will increase the catalytic exercise synergistically Extra file 1: Fig. S2 reveals the absorption peaks of varied formulations at 652 nm. Teams with out catalysts together with TMB + NaAc and TMB + H2O2 confirmed a weak absorption peak, whereas different response methods with completely different catalysts exhibited a powerful absorption peak at 652 nm. As well as, the simulated enzyme exercise of Fe3O4@MOF NPs was barely under that of the bare Fe3O4 NPs, which might be attributed to the adsorption of MOF on the floor of Fe3O4 NPs, resulting in the discount of catalytic centre. Nevertheless, the catalytic capability of FMA NPs was considerably enhanced by the Au NPs included in situ, which was attributed to the synergistic results of the mix of Fe3O4 NPs and Au NPs. The simulated enzymatic actions of catalysts at completely different concentrations had been additionally in contrast. The POD-mimicking actions of varied catalysts elevated with growing focus, and the absorbance of FMA NPs was the best, indicating the strongest POD actions (Fig. 3B).
Evaluation of the POD-mimicking catalytic actions and the steady-state kinetics. A Schematic illustrating the mechanism of enzymatic catalysis. B Catalytic actions of various nanoparticles with concentrations starting from 100 µg/mL to 400 µg/mL. C Catalytic efficiency of varied nanoparticles at various ranges of response pH. D Catalytic actions of various nanoparticles at various incubation temperatures. E Catalytic efficiency of various nanoparticles at various response instances. F and G Line weaver-Burk plots of the preliminary response fee versus the inverse of the substrate focus used to detect the kinetic parameters Okm and Vmax of FMA NPs with TMB or H2O2 as substrate
DMPO was used to find out the technology of radical species by trapping short-lived radicals to generate long-lived radical DMPO adducts, which had been examined utilizing ESR spectroscopy. The addition of hydrogen peroxide to the FMA NPs resolution induced the technology of enormous quantities of ·OH, which was confirmed by the precise ESR spectral peaks. As proven in Extra file 1: Fig. S3, the attribute spectrum of BMPO·OH was quadrilinear with an depth ratio of roughly 1:2:2:1. In distinction, no sign was noticed within the absence of added FMA NPs. The FMA NPs had been efficient in inducing the technology of ·OH radicals from H2O2 underneath acidic circumstances.
The catalytic efficiency of enzymes is influenced by the response time, temperature, and pH. Subsequently, the catalytic actions of various nanocomposites and pure POD had been decided underneath altering response circumstances. As proven in Fig. 3C, the FMA NPs exhibited steady catalytic actions inside a broad pH vary (from 3.0 to six.5), and the optimum response pH was 3.6. Determine 3D reveals that FMA NPs exhibit excessive catalytic actions between 20 and 50 °C, which overcomes the disadvantages of the pure temperature-sensitive POD. As proven in Fig. 3E, FMA NPs show considerably larger catalytic actions in the course of the 30 min response time in contrast with the opposite teams, which is attributed to the synergistic catalytic results of the mix of AuNPs and Fe3O4 NPs. Nevertheless, the actions of pure POD decreased after 15 min of response time. Thus, in contrast with pure POD, FMA NPs displayed excessive and steady POD-mimicking actions over a variety of pH circumstances, incubation temperatures, and response instances. The optimum response circumstances of FSC nanozymes had been pH 3.6, response temperature 37 °C, and a response time of 15 min.
Regular-state kinetics
The kinetic parameters of the hybrid FMA nanozymes had been decided underneath various TMB or H2O2 concentrations and optimum response circumstances. The outcomes confirmed that the catalytic response induced by FMA NPs fitted the everyday Michaelis-Menten mannequin (Fig. 3F and G). Thus, we used this mannequin to guage the catalytic capability of FMA nanozymes. The associated kinetic parameters (Okm and Vmax) had been derived from the Lineweaver-Burk plot. The Okm values had been 0.34 and 0.24 mM for TMB and H2O2, respectively, primarily based on Lineweaver-Burk double reciprocal plot (Fig. 3F and G). In contrast with different catalysts (Extra file 1: Desk S1), FMA nanozymes confirmed a bigger Vmax indicating wonderful catalytic effectivity. Underneath related catalyst concentrations, the worth of the catalytic fixed Okcat (Vmax / [E], wherein [E] is the focus of nanozyme or HRP) of FMA NPs was the most important, which signifies the best catalytic effectivity per nanoparticle.
Analysis of in vitro biosafety
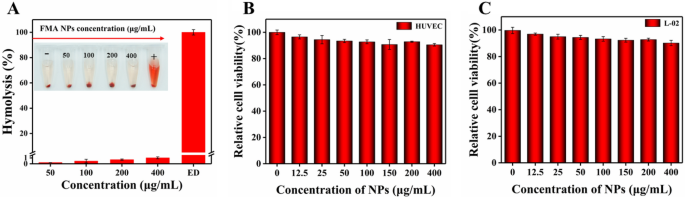
Nano-biomaterials exhibit wonderful biocompatibility for medical utility, and blood compatibility is a vital indicator of biocompatibility [50]. Subsequently, we investigated the hemolysis of FMA NPs. As proven in Fig. 4A, uncommon hemolytic results had been noticed underneath concentrations of 0-400 µg/mL. The hemolytic fee was effectively under 5%, indicating superior hemocompatibility on the given concentrations facilitating the antimicrobial utility of FMA NPs.
HUVECs and regular hepatocytes L-02 cells had been used to find out the in vitro cytotoxicity of FMA NPs. As proven in Fig. 4B and C, after therapy with FMA NPs for twenty-four h, the cell viabilities of HUVECs and regular hepatocytes L-02 cells had been better than 95% at low concentrations. The cell viabilities of HUVECs and hepatocytes L-02 cells remained above 90% even at FMA NP concentrations of 400 µg/mL, indicating the excessive biosafety of FMA NPs. Subsequently, the fabricated FMA NPs displayed wonderful biocompatibility and negligible toxicity.
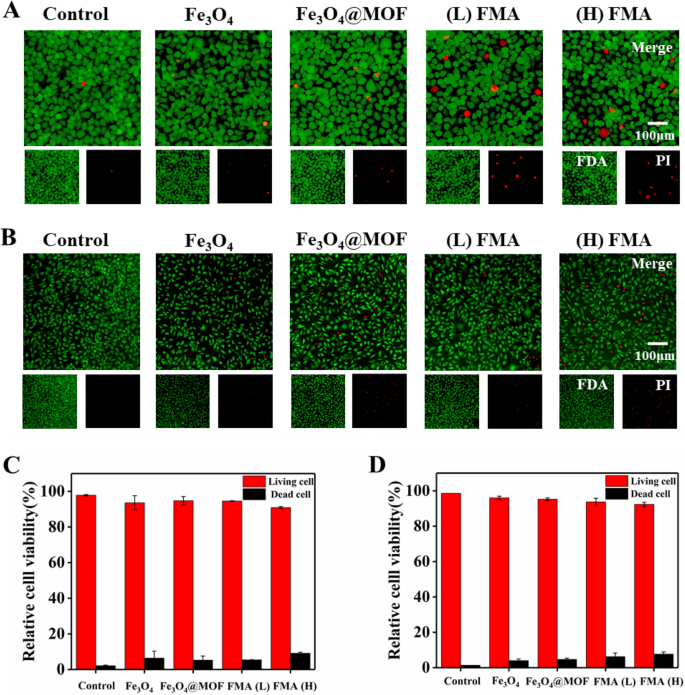
We additionally used fluorescence FDA/PI double labelling to evaluate the viability of L-02 cells and HUVECs after therapy with FMA NPs. The dwelling cells had been stained by FDA, and confirmed inexperienced fluorescence, whereas the lifeless cells had been stained by PI and displayed pink fluorescence [51]. As proven in Fig. 5A-B, in contrast with management, a small variety of cells had been lifeless after the co-incubation with completely different nanoparticles for 12 h. Nevertheless, the share of each stay cells was round 95% at 200 µg/mL of every nanoparticle. The viabilities of HUVECs and hepatocytes (L-02) had been 91% and 92%, respectively, even within the presence of 400 µg/mL of FMA NPs (Fig. 5C and D). These outcomes indicated that the fabricated nanoparticles displayed negligible toxicity.
Evaluation of the cell viability utilizing FDA/PI double labelling. A Staining of stay/lifeless hepatocytes L-02 cells co-cultured with completely different formulations. B Staining of stay/lifeless HUVECs co-cultured with completely different formulations. C Evaluation of relative viability of L-02 cells. D Evaluation of relative viability of HUVECs
Evaluation of antibacterial exercise in vitro
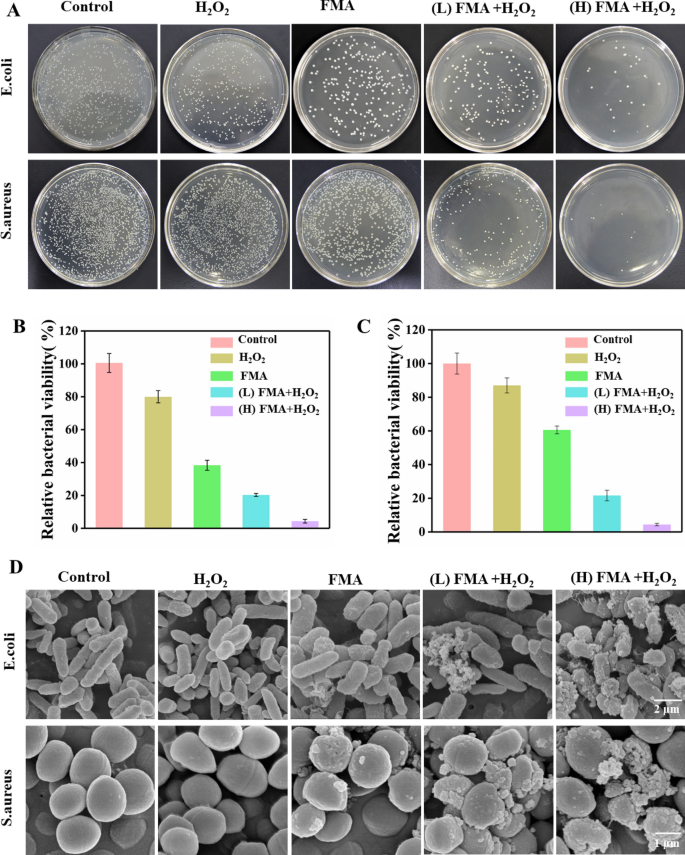
The antibacterial efficiency of FMA NPs was measured in vitro utilizing the plate counting technique. As proven in Fig. 6A, a slight lower was noticed within the variety of CFU following therapy with solely FMA NPs or H2O2 in contrast with the management, indicating that FMA NPs or H2O2 didn’t have a big impact on bacterial viability. In distinction, the therapy with FMA NPs and low-dose H2O2 had a outstanding inhibiting impact on bacterial progress. The antibacterial charges had been 76% and 72% for E. coli and S. aureus at low concentrations (200 µg/mL) of FMA NPs and H2O2, respectively. Particularly, the antibacterial actions of E. coli and S. aureus reached 97% and 98% following therapy with 400 µg/mL FMA NPs and H2O2, which was attributed to the manufacturing of ample poisonous·OH radicals. As proven in Extra file 1: Fig. S4, the MIC values instructed that therapy with the mix of FMA NPs and H2O2 resulted in elevated inhibition in opposition to E. coli and S. aureus. The MIC was achieved by growing the focus of FMANPs to 400 µg/mL, and when the focus reached 500 µg/mL, the inhibition of the 2 micro organism was better than 99%.
Morphological modifications in bacterial species after therapy
The morphological modifications of E. coli and S. aureus handled with completely different formulations had been decided by SEM. The untreated E. coli and S. aureus confirmed rod-like and spherical morphology with easy floor, respectively. When handled with H2O2, E. coli and S. aureus hardly ever displayed important modifications. Remedy with FMA NPs led to insignificant modifications within the total bacterial morphology, indicating a slight antibacterial exercise in opposition to E. coli and S. aureus. Co-treatment with excessive ranges of FMA NPs and H2O2 disrupted bacterial integrity and destroyed the bacterial morphology with unclear borders between micro organism, completely different levels of floor despair and dents because of the technology of poisonous ·OH (Fig. 6D). These outcomes had been in step with the colony-forming items, which had been additional confirmed by the improved antibacterial results of FMA NPs and H2O2.
Assay of antibacterial capability of FMA NPs in vitro.A Colonies of E. coli and S. aureus obtained after therapy with H2O2, FMA NPs, (low dose) FMA NPs + H2O2, and (excessive dose) FMA NPs + H2O2 are proven. B Relative exercise of E. coli in teams handled with completely different brokers. C Relative exercise of S. aureus in several therapy teams. D Typical SEM evaluation of E. coli and S. aureus uncovered to numerous remedies
Biofilm clearance
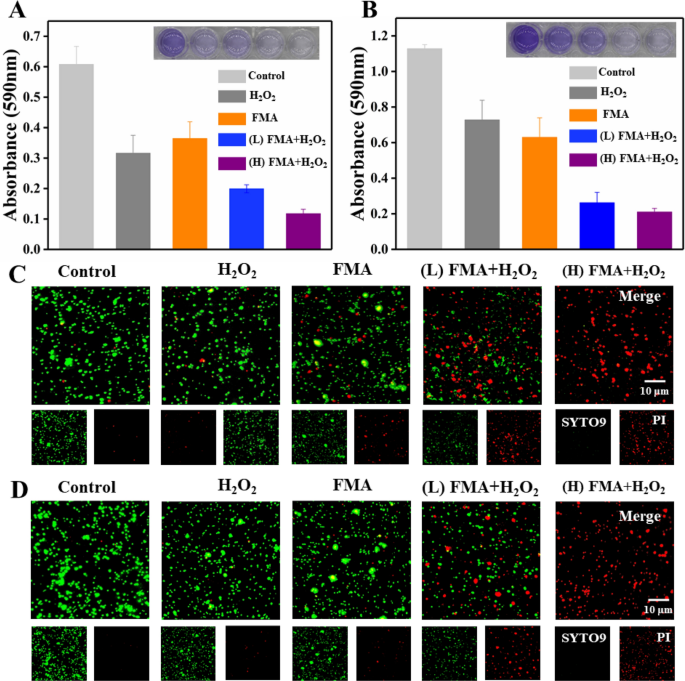
Biofilm formation was to analysed to find out the consequences of varied formulations utilizing crystalline violet staining. The biofilm was barely faraway from the underside of the 24-well microplates following therapy with FMA NPs or H2O2, whereas the therapy with the mix of FMA NPs and H2O2 led to important biofilm elimination. As proven in Fig. 7A and B, the therapy with excessive concentrations of FMA NPs (400 µg/mL) and H2O2 yielded the strongest anti-biofilm results, and the biofilm inhibition fee of E. coli and S. aureus reached 83% and 91%, respectively. We additionally investigated the ring-breaking capability on biofilms by incubating completely different formulations with pre-formed biofilms. In contrast with management biofilms, which had been barely cleared by therapy with H2O2 and FMA NPs, the inhibition of E. coli and S. aureus reached 75% and 85% following therapy with FMA NPs and H2O2 (Extra file 1: Fig. S5A and B). These outcomes additionally demonstrated apparent enhancement within the antibacterial actions of FMA NPs and H2O2.
A Disruption of E. coli biofilms in numerous therapy teams. B Biofilm disruption impact on S. aureus in numerous therapy teams. C Fluorescence photos of stay/lifeless E. coli (inexperienced/pink) with completely different remedies. D Fluorescence photos of stay/lifeless S. aureus (inexperienced/pink) underneath completely different remedies
Evaluation of stay and lifeless micro organism
To additional examine the improved antibacterial talents of FMA NPs, nucleic acid dyes SYTO9 and PI had been employed in stay/lifeless cell assays. As proven in Fig. 7C and D, the inexperienced fluorescence alerts of E. coli and S. aureus had been predominant within the management group, which indicated that nearly no lifeless micro organism. Therapies with H2O2 or FMA NPs revealed restricted pink fluorescence because of the low antibacterial effectivity. In contrast, co-treatment with H2O2 and FMA NPs considerably elevated the variety of lifeless micro organism because of the manufacturing of extremely poisonous ·OH radicals as proven by the predominant pink fluorescence sign, which instructed that the antibacterial effectivity was significantly enhanced and resulted in intensive bacterial dying (Fig. 7C and D). Publicity to excessive concentrations of FMA NPs and H2O2 led to the disappearance of the inexperienced fluorescence sign, indicating that the bactericidal capability trusted the focus of FMA NPs.
Analysis of wound-healing results
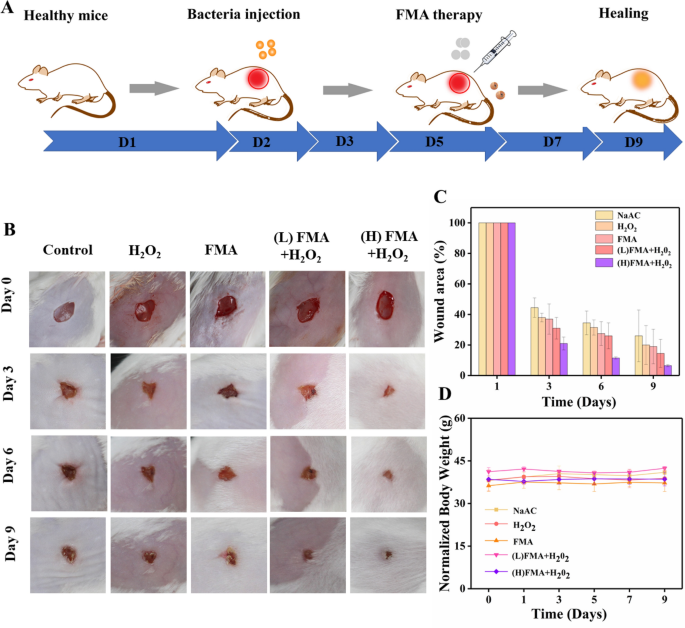
Given the superb antibacterial results noticed, the potential wound-healing results of FMA NPS in vivo had been investigated for KM mice. 5 teams of random mouse fashions had been developed by producing dorsal pores and skin wounds. Subsequently, bacterial wound infections had been created by exposing the injuries to 107 CFU/mL S. aureus. Varied formulations had been injected into the wound after 1-day an infection. Determine 8A reveals the therapy protocol within the animal mannequin. As proven in Fig. 8B, a comparatively gradual wound therapeutic was detected within the management, and after therapy with H2O2 or FMA NPs, which was attributed to the low catalytic capability. In contrast, the wound therapeutic fee reached greater than 80% by day 3 post-injection when handled with FMA NPs and H2O2. The wound crusted and accomplished healed after 9 days post-injection, and the therapeutic fee was remarkably larger than within the different teams (Fig. 8C), indicating the wound-healing results of ·OH radicals. Considerably, the therapy with FMA NPs and H2O2 considerably accelerated the therapeutic course of in contrast with the management. Extra importantly, the physique weight of the handled mice didn’t change clearly, indicating the absence of any poisonous unintended effects related the designed FMA nanozymes in vivo (Fig. 8D).
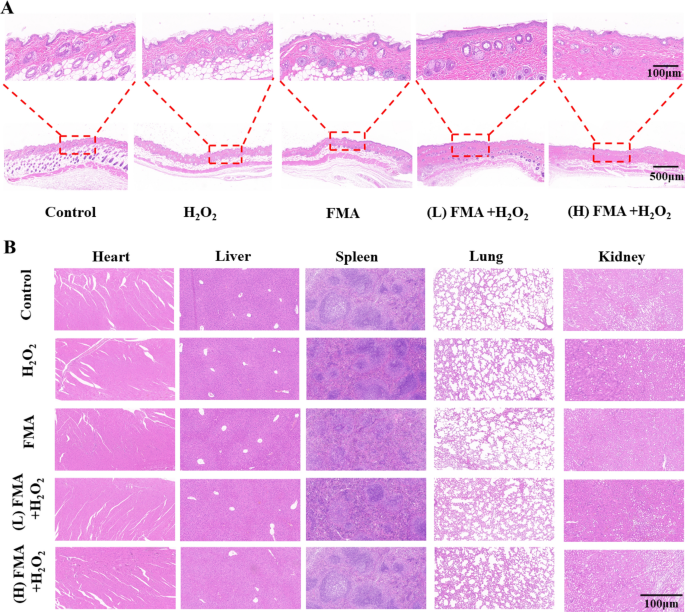
To evaluate the wound-healing effectivity in numerous mice, hematoxylin and eosin (H&E) staining was carried out for histological evaluation of the wound tissues. As proven in Fig. 9A, in contrast with decreased wound closure with incomplete epidermal layers and inflammatory cells noticed after H2O2 therapy, an intact epidermal layer within the wounds was noticed after therapy with FMA NPs and H2O2, and the mice displayed a excessive diploma of wound re-epithelialisation when handled with excessive concentrations. Thus, the ready hybrid FMA nanozymes displayed a wonderful wound-healing capability in vivo. Additional, the H&E staining of the important thing organs within the handled mice displayed no lesions, indicating the biosafety of FMA nanozymes in vivo (Fig. 9B).
[ad_2]