
[ad_1]
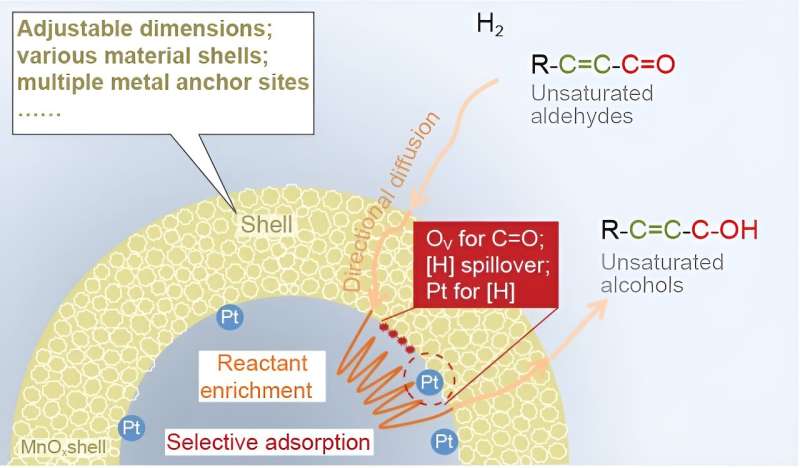
Hole-structured supported steel catalysts (i.e. nanoreactor catalysts) with encapsulated lively websites and well-defined shells present an excellent place for multicomponents to react or remodel cooperatively in an orderly method, and effectively have been acknowledged as probably the most well-liked catalyst candidates.
Though reactant enrichment has been proposed by investigating the connection between the catalytic efficiency and the construction of nanoreactors on the nano stage, the examine of the enrichment impact on the mesoscale (500-2000nm) remains to be not complete sufficient. Establishing the nanoreactor fashions with lively metals inside and out of doors the hole nanostructure by way of totally different artificial strategies or sequences will inevitably influence the microenvironment across the lively websites, in addition to the important lively websites.
Moreover, reactant enrichment on the mesoscale stage entails many processes reminiscent of adsorption and diffusion, which can’t be elaborated by setting up easy computational fashions on the nanoscale stage. Subsequently, investigation of the reactant enrichment on the mesoscale stage requires sustaining the intrinsic lively websites fixed when setting up the analysis mannequin, both with or with out enrichment habits.
In a brand new analysis article printed in Nationwide Science Overview, scientists at Dalian Institute of Chemical Physics (DICP), College of Chinese language Academy of Sciences, Taiyuan College of Know-how, College of Surrey, and Internal Mongolia College current a brand new nanoreactor catalyst (Pt NPs@MnOx ) with uniformly dispersed Pt nanoparticles encapsulated in an oxygen vacancy-rich MnOx hole construction to catalyze the selective hydrogenation of CAL and examine reactant enrichment on the mesoscale stage.
The catalytic efficiency for CAL-selective hydrogenation on Pt NPs@MnOx is 3.4-fold increased than that of Pt NPs&MnOx, which is bodily crushed into an open construction. UV–vis, in situ FTIR and IGA measurements show that the hole MnOx shell of Pt NPs@MnOx results in increased CAL uptake.
The mechanism behind this phenomenon might encompass two steps. Because the hole construction creates a confined area, outer reactants would constantly diffuse into the inside of the hole construction directionally pushed by the focus gradient and/or capillary-like impact (step 1).
Then, these reactants are mounted on the inside floor by adsorption to maintain the native low focus within the confined area. In distinction, Pt NPs&MnOx couldn’t assist this directional diffusion course of. Furthermore, DFT outcomes reveal that CAL is extra strongly adsorbed on the floor of Pt NPs@MnOx than Pt NPs&MnOx below extra reactants (step 2).
H2-TPR–MS and finite-element simulation outcomes additionally show that the Pt NPs@MnOx nanoreactor creates a steady area with a excessive focus and low circulation fee to stop the escape of the reactants (dissociated hydrogen). It’s due to this fact clear that reactant enrichment is derived from the directional diffusion of reactant pushed by way of an area focus gradient and an elevated quantity of reactant adsorbed because of the enhanced adsorption capability in hole MnOx.
The Pt NPs@MnOx catalyst displays extraordinarily excessive catalytic actions and selectivity in a variety of response pressures. A 95% conversion with 95% COL selectivity is obtained on Pt NPs@MnOx at solely 0.5 MPa H2 and 40 min, which is a comparatively delicate situation in contrast with most reported catalytic programs.
Combining experimental outcomes with density purposeful principle calculations, the superior cinnamyl alcohol (COL) selectivity originates from the selective adsorption of CAL and the fast formation and desorption of COL within the MnOx shell. Furthermore, the hole void induces the reactant-enrichment habits, enhancing the response exercise.
These findings supply the opportunity of enhancing the catalytic efficiency on the mesoscale stage by designing a rational nanoreactor, relatively than lowering the scale of the steel particles or modifying them with heteroatoms or ligands on the nanoscale stage.
Extra data:
Yanfu Ma et al, Reactant enrichment in hole void of Pt NPs@MnOx nanoreactors for reinforcing hydrogenation efficiency, Nationwide Science Overview (2023). DOI: 10.1093/nsr/nwad201
Supplied by
Science China Press
Quotation:
Reactant enrichment of nanoreactors boosts hydrogenation efficiency (2023, November 1)
retrieved 1 November 2023
from https://phys.org/information/2023-11-reactant-enrichment-nanoreactors-boosts-hydrogenation.html
This doc is topic to copyright. Aside from any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.
[ad_2]